Abstract
Purpose
Materials and Methods
Results
Notes
Ethical Statement
The institutional review board of each institution approved this study (approval number: H-2005-179-1126 at Seoul National University Hospital), and waived the requirement for obtaining informed consent.
Author Contributions
Conceived and designed the analysis: Kim K, Shin KH.
Collected the data: Kim K, Jung J, Kim H, Jung W, Shin KH, Chang JH, Kim SS, Park W, Chang JS, Kim YB, Ahn SJ, Lee IK, Lee JH, Park HJ, Cha J, Kim J, Choi JH, Koo T, Kwon J, Kim JH, Kim MY, Park SH, Kim YJ.
Contributed data or analysis tools: Shin KH.
Performed the analysis: Kim K.
Wrote the paper: Kim K, Jung J, Kim H, Jung W, Shin KH, Chang JH, Kim SS, Park W, Chang JS, Kim YB, Ahn SJ, Lee IK, Lee JH, Park HJ, Cha J, Kim J, Choi JH, Koo T, Kwon J, Kim JH, Kim MY, Park SH, Kim YJ.
References
Fig. 1
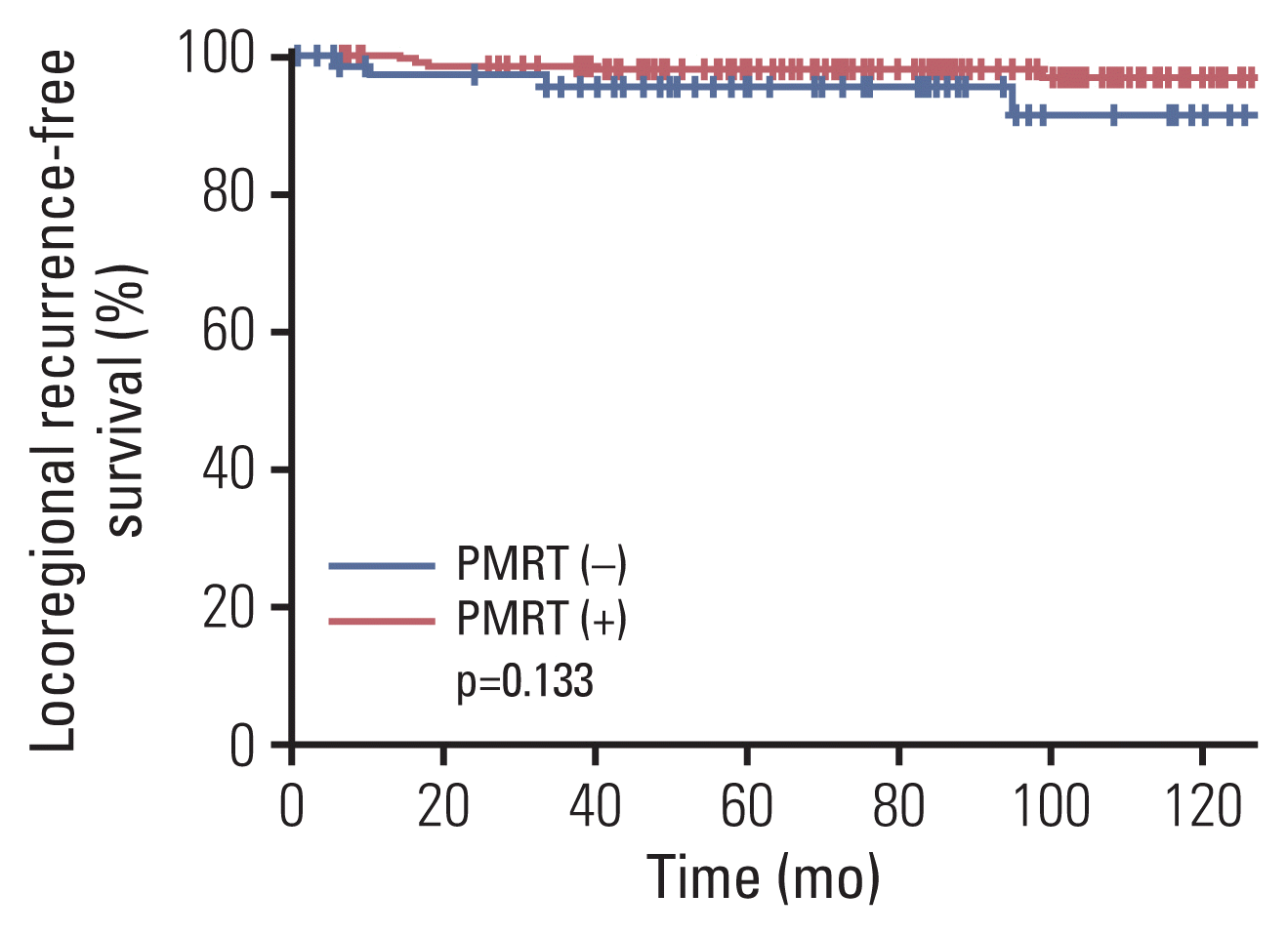
Fig. 2
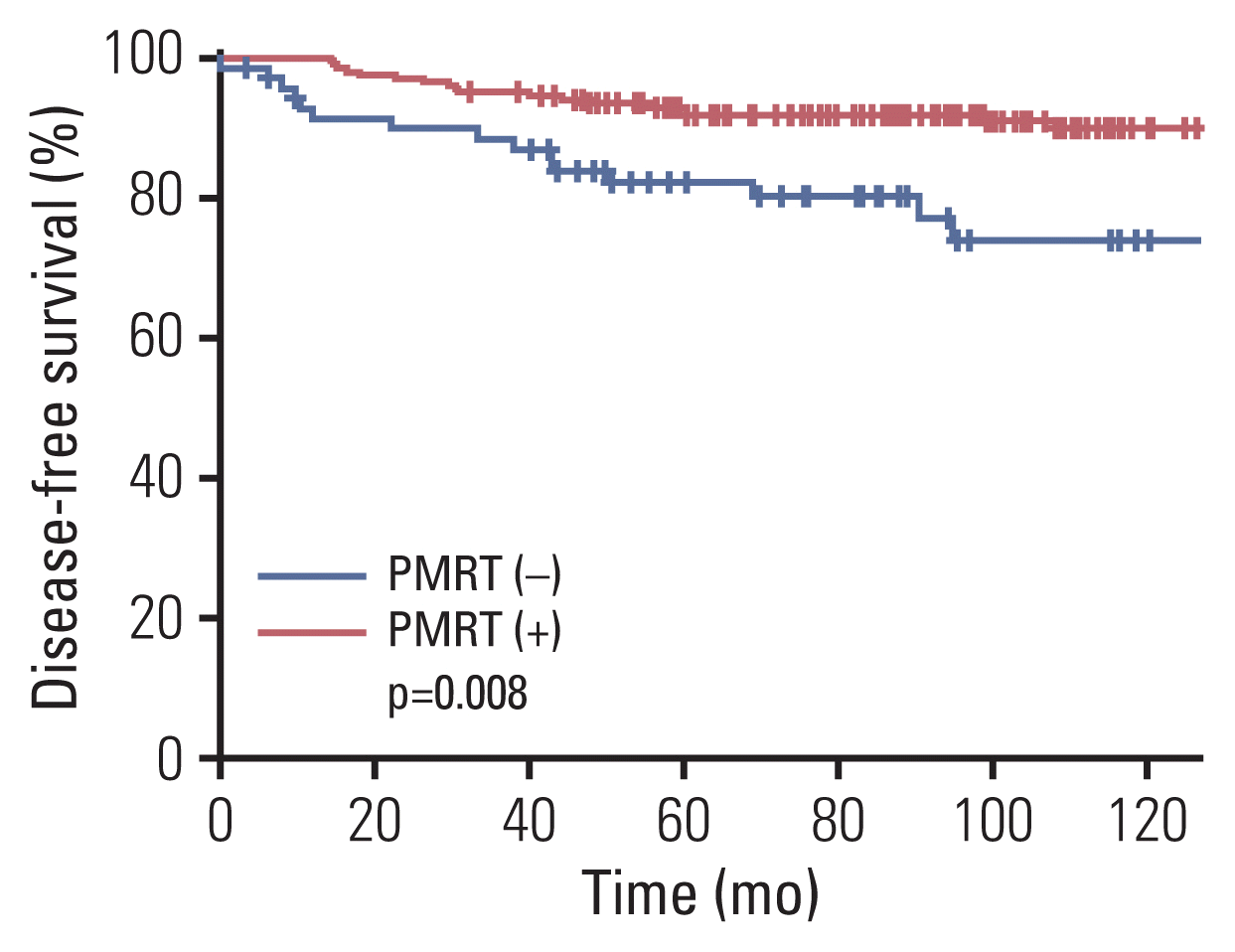
Table 1
Table 2
| No. | Age (yr) | Tumor size (cm) | ER | PR | HER2 | HG | LVI | RM | Chemotherapya) | Hormonal therapyb) | PMRT | LRR site | Time-to LRR (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 5.2 | Neg | Neg | Neg | 3 | Pos | Neg | Yes | No | Yes | SCL (out-field) | 14 |
| 2 | 33 | 7 | Pos | Neg | Neg | Unknown | Neg | Neg | Yes | Yes | Yes | Ax (in-field) | 97 |
| 3 | 74 | 6.3 | Neg | Neg | Neg | 3 | Neg | Neg | Yes | No | Yes | SCL (out-field) | 17 |
| 4 | 35 | 5.2 | Pos | Neg | Neg | 2 | Pos | Neg | Yes | Yes | Yes | SCL/IMN (out-field) | 39 |
| 5 | 49 | 5.8 | Neg | Neg | Neg | 3 | Pos | Neg | Yes | No | Yes | Ax (in-field) | 16 |
| 6 | 42 | 11 | Neg | Neg | Neg | Unknown | Unknown | Neg | Yes | No | No | Ax | 6 |
| 7 | 45 | 5.5 | Pos | Pos | Pos | 2 | Neg | Neg | Yes | Yes | No | CW | 32 |
| 8 | 43 | 5.5 | Neg | Neg | Neg | 3 | Neg | Neg | Yes | No | No | CW | 10 |
| 9 | 61 | 5 | Pos | Pos | Pos | 3 | Pos | Neg | No | No | No | SCL/IMN | 93 |
Ax, axillary node; CW, chest wall; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HG, histologic grade; IMN, internal mammary node; LRR, locoregional recurrences; LVI, lymphovascular invasion; Neg, negative; PMRT, postmastectomy radiation therapy; Pos, positive; PR, progesterone receptor; RM, resection margin; SCL, supraclavicular node.
Table 3
Table 4
Table 5
| Author (yr) | PMRT | No. of patients | LRR | DFS | OS | |||
|---|---|---|---|---|---|---|---|---|
| Taghian et al. (2006) [9] | No | 313 | 10.0% (10 yr) | NA | NA | |||
| Floyd et al. (2006) [10] | No | 70 | 7.6% (10 yr) | 82% (10 yr) | 72% (10 yr) | |||
| Goulart et al. (2011) [14] | No | 56 | 8.9% (10 yr) | p=0.200 (univariate) | 74.6% (CSS, 10 yr) | p=0.200 (univariate) | NA | - |
| Yes | 44 | 2.3% (10 yr) | 85.8% (CSS, 10 yr) | NA | ||||
| Johnson et al. (2014) [6]a) | No | 1,462 | NA | - | 82.4% (CSS, 8 yr) | p=0.045 (multivariate) | 61.8% (8 yr) | p < 0.001 (multivariate) |
| Yes | 1,063 | NA | 85.0% (CSS, 8 yr) | 76.5% (8 yr) | ||||
| Francis et al. (2017) [7]a) | No | 1,400b) | NA | - | NA | - | 59.2% (10 yr) | p < 0.001 (univariate) |
| Yes | 1,400b) | NA | NA | 67.4% (10 yr) | ||||
| This study | No | 72 | 8.7% (8 yr) | p=0.133 (univariate) | 73.9% (8 yr) | p=0.009 (multivariate) | NA | - |
| Yes | 202 | 2.0% (8 yr) | 91.8% (8 yr) | NA | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download