Introduction
Melanoma is the most aggressive skin cancer and is the sixth most frequently diagnosed cancer in the United States in both sexes [
1]. The incidence of melanoma increases because of the gradually more aging society, better and earlier detection of melanoma using screening programs, and enhanced public awareness [
1]. For Caucasians, the annual increase in the incidence rate of melanoma has been estimated to be approximately 3%–7% each year [
2]. Melanoma incidence rates vary depending on ethnicity. In the United States, Hispanics (4.5/100,000), and blacks (1.0/100,000) have a lower incidence than non-Hispanic whites (21.6/100,000) [
3]. Melanoma was detected in 1.2 million people in the United States in 2015, and based on data for 2013–2015, 2.3% of the US population will be diagnosed with melanoma over their lifetime [
4]. Melanoma has a substantially lower incidence than basal cell carcinoma or squamous cell carcinoma. However, it has a much worse prognosis [
1]. The survival rate of melanoma differs according to the pathological stage. Patients in stages I and II have a nearly 100% 5-year survival rate, whereas just 36% of patients diagnosed with stage IVa have a 5-year survival rate [
5].
Melanoma incidence and prevalence trends appear to be consistent worldwide, with only variations when comparing latitudes. However, specific geographic locations are related to ultraviolet (UV) exposure. Caucasians are susceptible to cutaneous melanoma development, with the magnitude of risk depending on sun exposure patterns (intermittent or cumulative UV exposure) and individual inherited genetic factors [
6]. Consequently, by far the highest incidence in both men and women is in Australia and New Zealand [
7]. However, only limited information is available on the trends in cutaneous melanoma incidence and survival among East Asians, reportedly having a lower incidence rate than Caucasians [
8].
South Korea has a public medical insurance system known as the National Health Insurance (NHI) program. NHI claims data are obtained from payment claims generated during patient visits to each medical institution. NHI claims data include diagnosis, treatment, procedure, medication, and survival information with general demographics including patient’s age, sex, area of residence, and income. This system covers more than 50 million people (98% of the whole population of South Korea) [
9].
Therefore, this study aimed to investigate the patterns of melanoma incidence, prevalence, and survival rate in South Korea in terms of various demographic factors using NHI claims data from 2004 to 2017.
Discussion
This study showed the nationwide trends of melanoma incidence and survival rate by age, sex, income, area of residence, and anatomical sites using NHI claims data from 2004 to 2017. We used NHI claims data, KOSIS, KMA, and Korea Health Promotion Institute data for comprehensively studying melanoma epidemiology. To our knowledge, this was the first study to use such varied types of nationwide data.
We found that the incidence of melanoma gradually increased from 2004 to 2017 at 3.0/100,000/yr in 2017. The incidence has increased in most countries, although in Asian countries, it remains relatively low [
17]. Among 100,000 people, the incidence was reported at 1.75/100,000/yr in Japan, 0.65/100,000/yr in Taiwan, and 8.0/100,000/yr in China [
18]. Australasian, North American, European populations were most greatly burdened by melanoma, especially older people, at 54/100,000/yr in Australasia, 21/100,000/yr in North America, 16/100,000/yr in Western Europe, 8/100,000/yr in Central Europe, and 8/100,000/yr in Eastern Europe [
19]. We suspect that the increased incidence of melanoma may be related to the development of diagnostic technologies and increased public awareness. It can be assumed that the increase in outdoor leisure activities and the westernization of lifestyles have also had an effect.
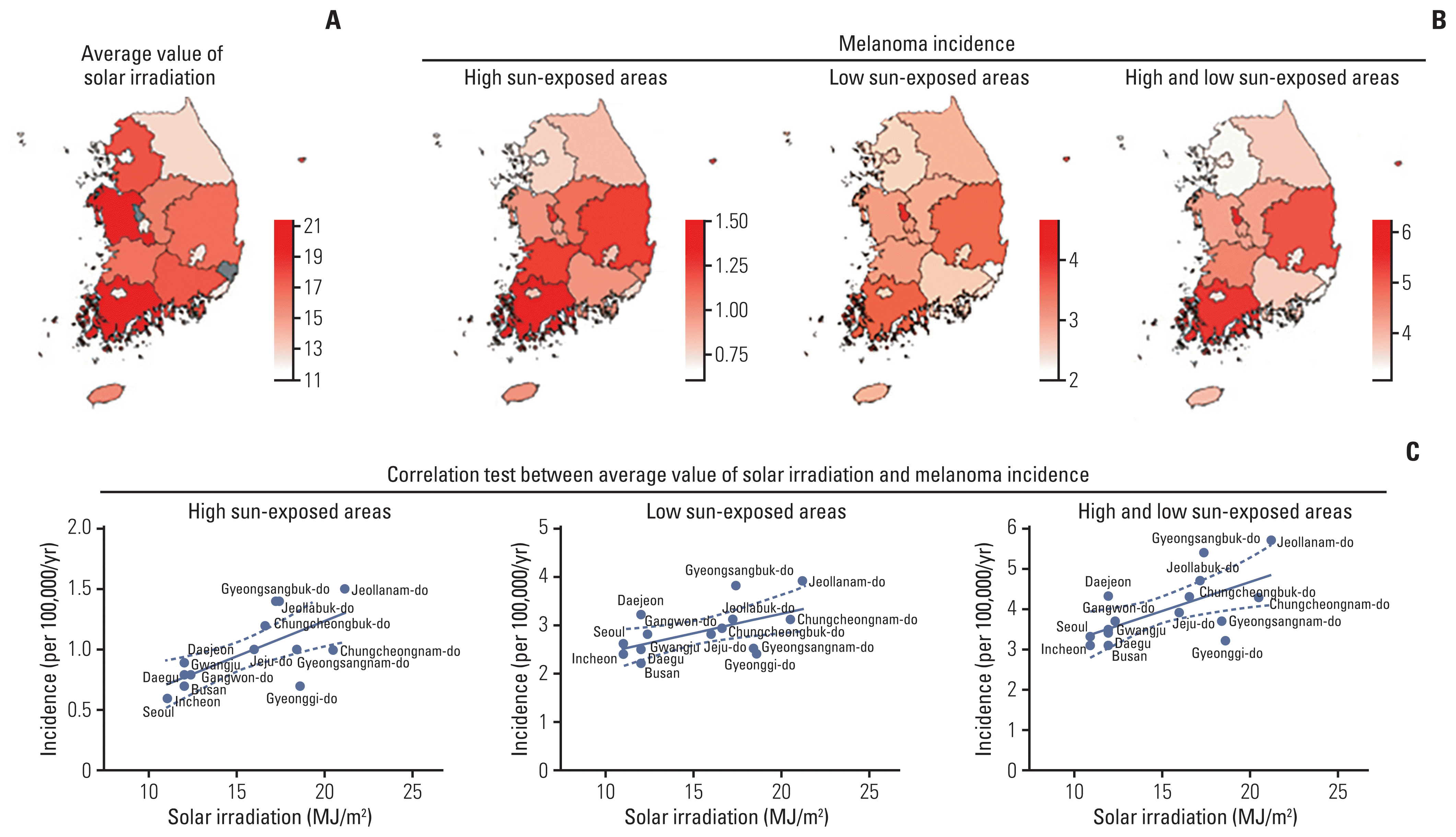
We classified and analyzed patients with melanoma according to their exposure site to sunlight. We observed that low sun-exposed sites were more greatly affected by melanoma than high sun-exposed sites in South Korea. Previous studies have reported similar results. Shaikh et al. [
20] found 19,900 patients diagnosed with melanoma in the face and neck and 75,696 patients with melanoma in the trunk and limbs in the United States from 1989 to 2009. Likewise, Tomizuka et al. [
18] also reported that melanoma in the trunk and limbs was more prevalent than melanoma in the head and neck in Japan (3,505 vs. 280).
Sunlight exposure and the increased likelihood of developing skin cancer have been a frequent subject of research. Parkin et al. [
2] reported that approximately 86% of melanoma was associated with UV exposure. The risk for melanoma doubled after just one blistering sunburn during childhood [
21]. Irrelevant by anatomical site, we found a significant correlation between incidence of melanoma and amount of solar irradiation. Interestingly, our results showed statistically significant in the melanoma patients of low sun-exposed sites (
Fig. 1C). The exact reason for these results cannot be fully elucidated in this study. However, we speculate that physical stress, such as trauma and genetic factors, and intermittent-sun exposure may be associated complexly in melanoma development [
22–
24]. Several studies have reported that the association between melanoma and sun exposure may differ according to anatomical sites. Caini et al. [
7] has reported that melanoma on the head and neck was more greatly related to continuous sun exposure, whereas melanoma on the trunk and limbs was associated with intermittent-sun exposure patterns. Gandini et al. [
25] performed the meta-analysis about the risk factors for melanoma and concluded that intermittent-sun exposure was positively associated with melanoma, but the high continuous pattern of sun exposure had the inverse association. Additionally, we obtained global UV radiation level and melanoma incidence data from the World Health Data Platform and International Agency for Research on Cancer, respectively [
26], and compared the UV radiation dose and worldwide incidence of melanoma. However, there was no significant correlation globally (
S4 Fig.). We speculate that it is because these data from various countries are not standardized and are not analyzed by the anatomical site of the melanoma. Furthermore, genetics, ethnicity, and other factors are also important.
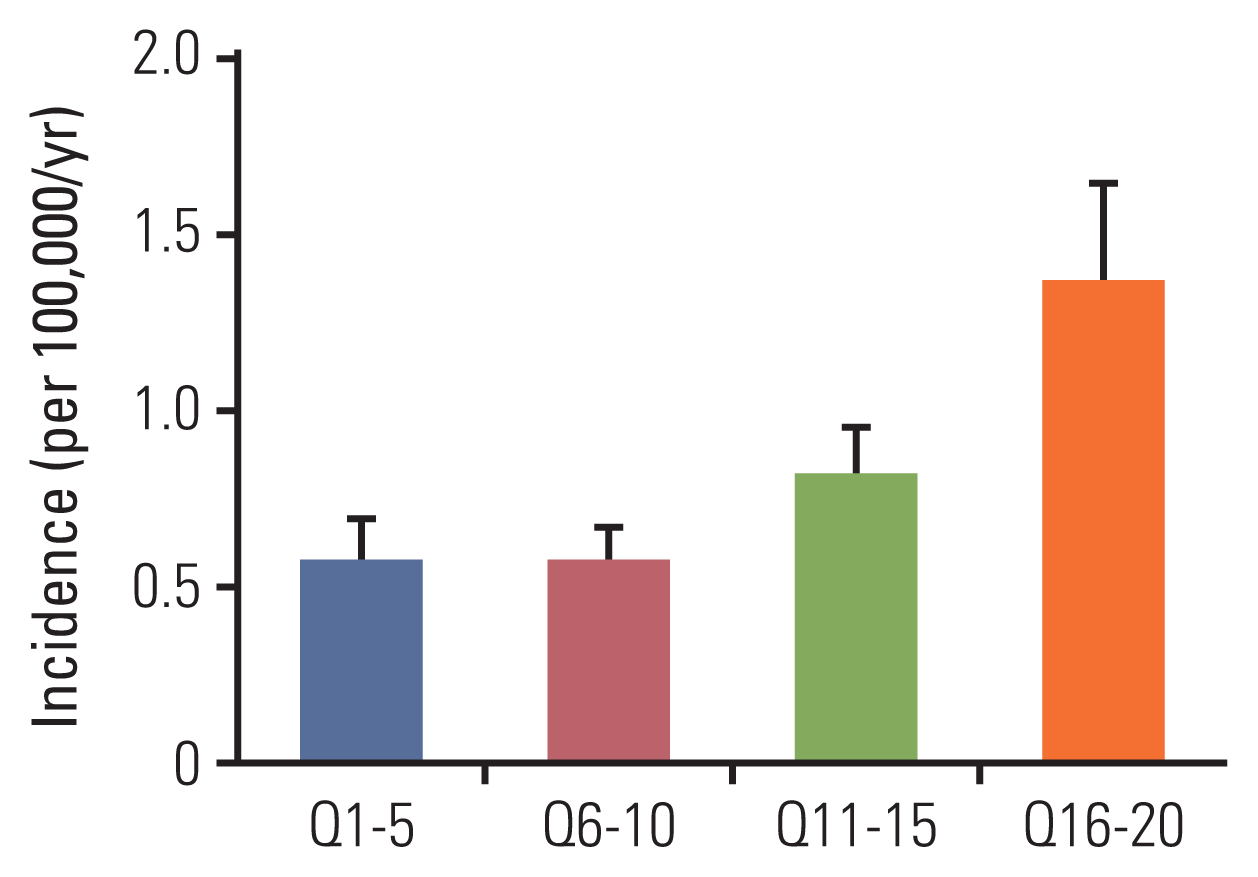
Using the NHI claims data, income could be precisely estimated because the National Health Insurance premium is based on household income [
9]. It was found that the top 25% of people had approximately 0.79 higher age-standardized melanoma incidence compared to the bottom 25% (
Fig. 2). Previous studies have shown that melanoma incidence was higher among people in high socioeconomic status groups in Canada and the Netherlands [
27,
28]. We believe that it is because the higher the income, the higher the awareness of healthcare. Studies have also shown that awareness of, and access to, screening for cancer is disproportionately higher in individuals with higher socioeconomic status [
29].
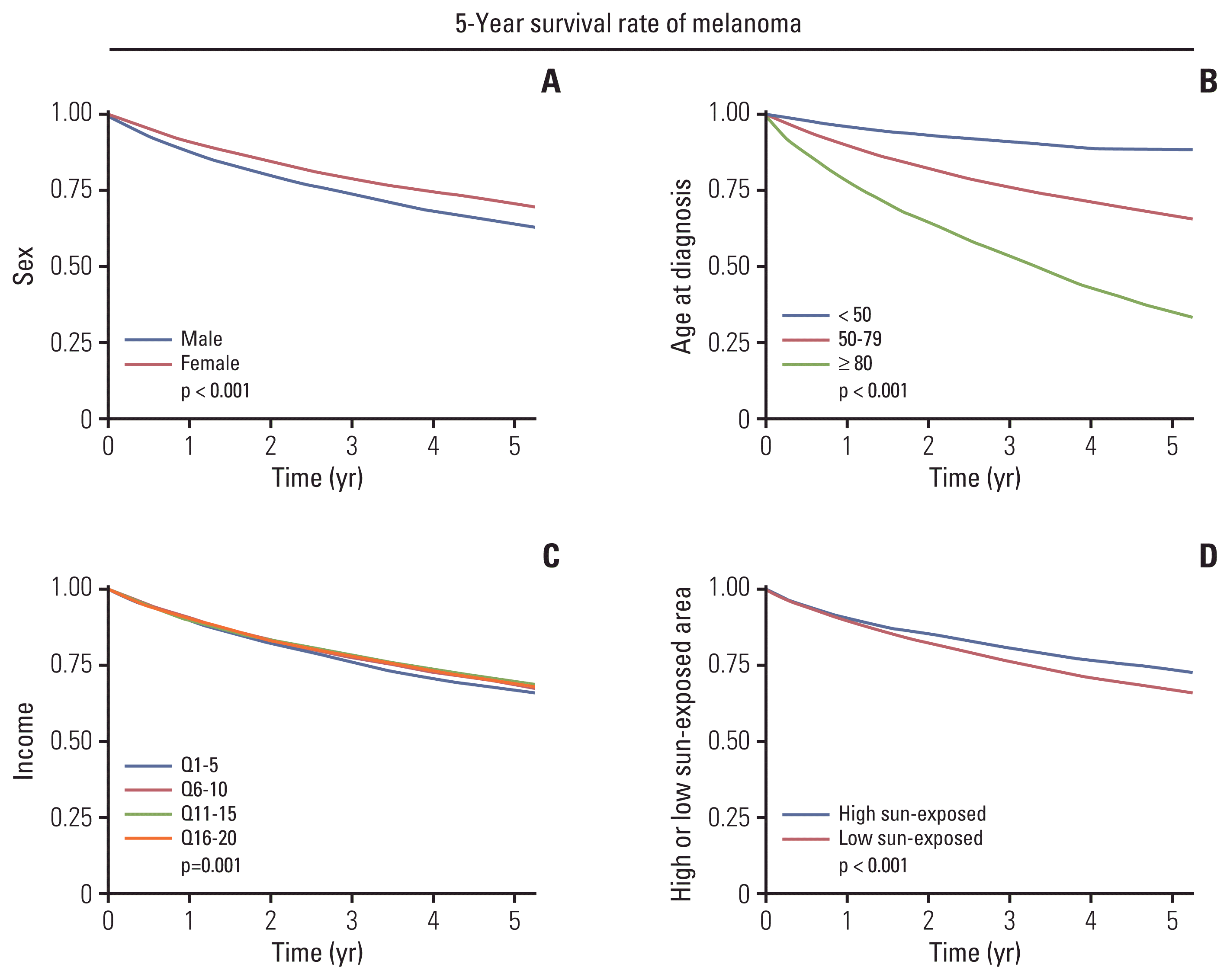
We found that being male, lower income, and melanoma at low sun-exposed sites had the lowest survival rate, although the differences only ranged from 2.0% to 5.6% for each variable. We cannot conclude that these results are truly significant because the p-value became extremely low because of the large sample size in the analyses of nationwide data [
30].
Some studies have reported differing views on the melanoma prognostic impact according to income. Geller et al. [
31] investigated melanoma mortality among the socioeconomically disadvantaged, reporting that those with lower socioeconomic status may be more likely to die than patients of higher socioeconomic status. Hopkins et al. [
32] concluded that average household income and education levels were not associated with melanoma survival.
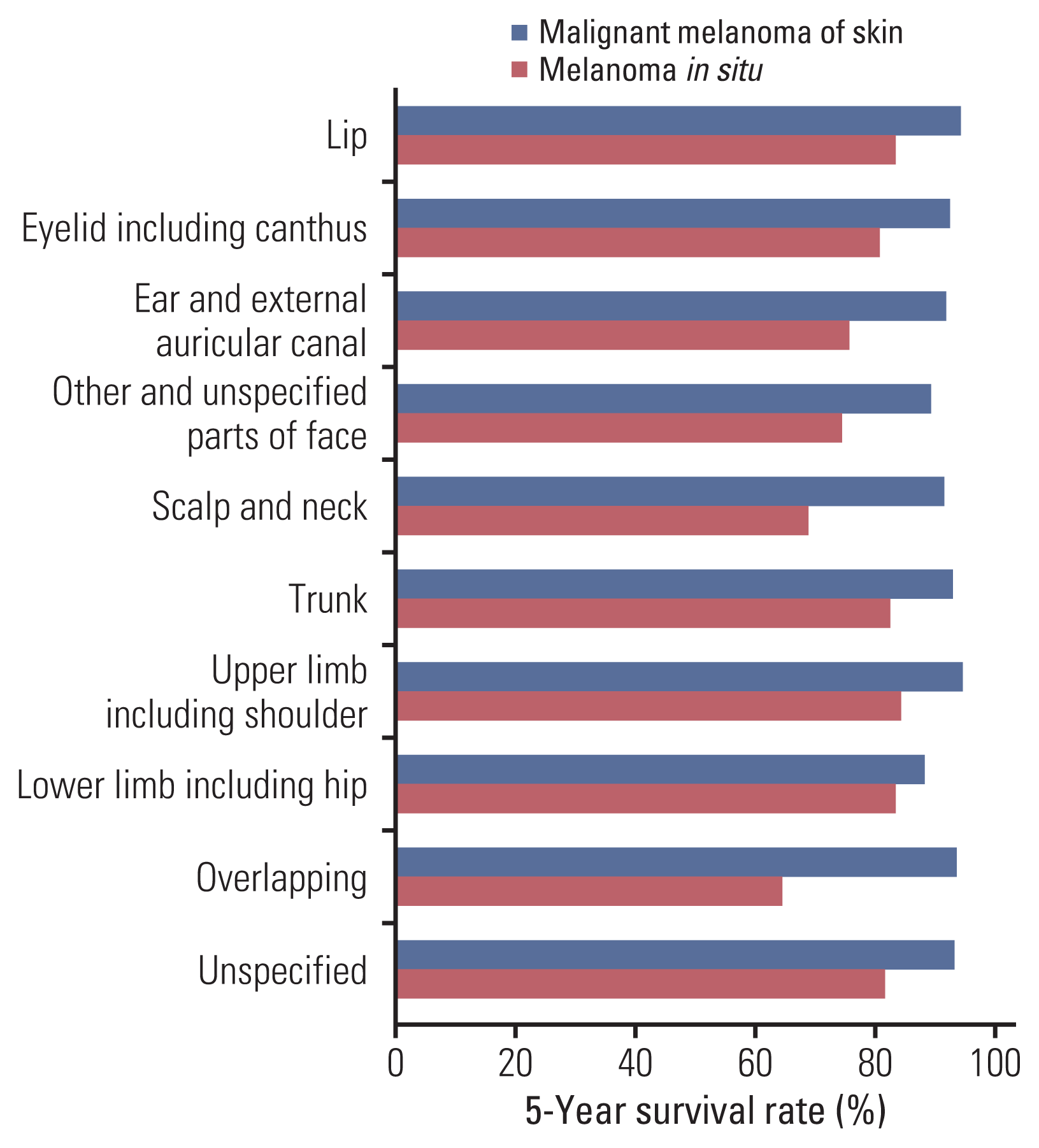
The relationship between melanoma prognosis and anatomical sites is also controversial for each study. Gillgren et al. [
33] concluded the survival rates according to anatomical sites, by the following order, starting with the lowest neck-trunk and foot, face, and lower and upper limbs. Howard et al. [
34] reported that the dorsal shoulder, superior back, and clavicular sites showed a worse prognosis than the other anatomical regions, including the calves, Achilles, upper arms, forehead, temples, cheeks, and face. A further prospective study will be necessary to elucidate these. On the other hand, Berwick et al. [
35] reported that intermittent-sun exposure might increase survival from melanoma. Holidays with sun exposure were associated with favorable melanoma prognostic factors.
Nevertheless, in the case of sex, there is evidence that relative survival remains worse for men. Balzi et al. [
36] reported that women had a clear prognostic advantage over men. Sharouni et al. [
37], likewise, demonstrated that after adjustment for all other variables (age, breslow thickness, localization, ulceration, and morphological subtype), sex remained significantly associated with a higher risk of dying among men with a relative excess risks for men at 1.37.
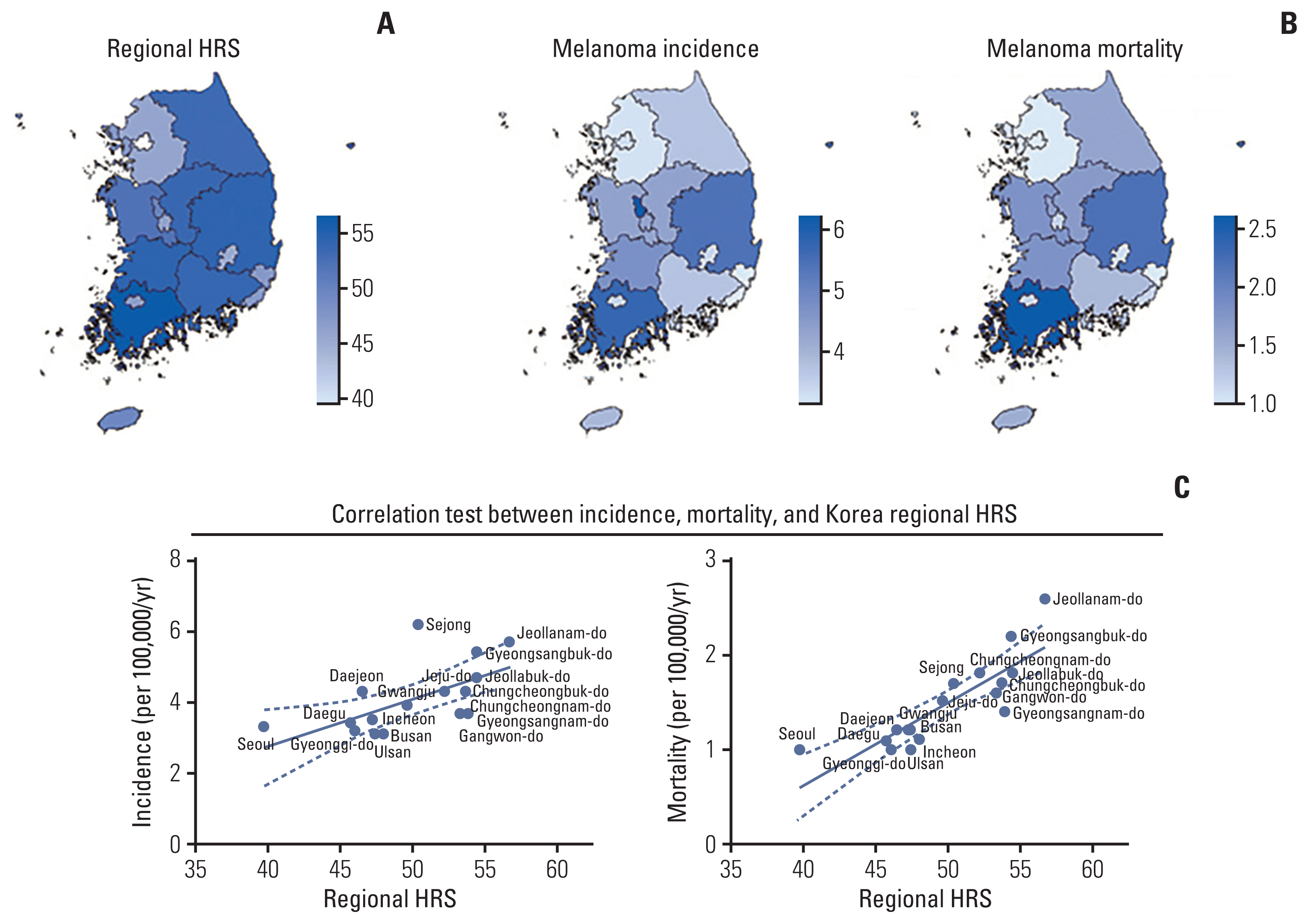
Subsequently, we assumed that a regional medical service gap would influence the incidence or mortality of melanoma. To evaluate the medical service gap, we used the Korea regional HRS obtained from the Report for Healthcare Vulnerable Region of Korea 2016 published by the Korea Health Promotion Institute [
15]. Results showed a high positive correlation between the Korea regional HRS and both melanoma incidence and mortality. It should be noted that these regional differences will be helpful for the nationwide distribution of medical resources and services in the future.
This study had both strengths and limitations. This study was based on the real-world data of an NHI claims database in South Korea. We collected the long-term data of more than 25,000 patients who had melanoma without selection bias. South Korea has a public health insurance system that covers the entire population and a copayment decreasing policy for RIDs such as melanoma. Therefore, all patients with melanoma were registered in the healthcare system, and their data could be examined. However, this study involves the potential limitations of a retrospective observational design. The NHI claims data did not include information on possible confounding factors such as radiologic and laboratory findings, and the etiologic mechanism of melanoma. Acral melanoma, the most common subtype of melanoma in South Korea, occurs on the palm, sole, and nails (non-sun exposure sites). It does not include the thigh, calf, shin, arm, and forearm (intermittent-sun exposure sites). In NHI claims data, extremities codes do not divide acral site from extremities. Hence, we could not perform subgroup analysis according to the pathological melanoma subtype. The NHI claims data also did not include individual patient’s information such as occupation, lifestyle, and habits. For this reason, the association between patient’s way of life and solar irradiation was not considered. A possible explanation of better survival in the high sun-exposed sites (head and neck melanoma) is the earlier diagnosis because this site is more visible than low sun-exposed melanoma. In multiple linear regression analysis, we only included SID and HRS as independent variables because the other variables such as age, sex, income, and area of residence were not linked with the SID and HRS.
The incidence of melanoma in South Korea is increasing. Sun exposure might be an important pathogenic factor in South Korea for melanoma development in the melanoma patient of high sun-exposed sites. Regions with poor medical services or accessibility had higher incidence and lower survival of melanoma. Our study provides valuable data, which will be helpful for consultations and for establishing healthcare systems for patients with melanoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download