Abstract
Purpose
Poor oral health is associated with head and neck cancer (HNC). We evaluated whether a national oral health screening program (OHSP) could reduce the risk of HNC.
Materials and Methods
Data from 408,247 healthy individuals aged ≥ 40 years from the National Health Insurance System-National Health Screening program during 2003 and 2004 in Korea were analyzed. The risk of HNC was compared between subjects who underwent OHSP (HEALS-Dental+, n=165,292) and routine health check-ups only (HEALS-Dental−, n=242,955). The impact of individual oral health-related factors on HNC risk was evaluated in HEALS-Dental+.
Results
A total of 1,650 HNC cases were diagnosed. The 10-year HNC-free rate was 99.684% with a median follow-up of 11 years. The risk of all HNC (hazard ratio [HR], 1.16; 95% confidence interval [CI], 1.03 to 1.29; p=0.011) and oropharyngeal cancer (HR, 1.48; 95% CI, 1.13 to 1.94; p=0.005) was significantly higher in HEALS-Dental− than in HEALS-Dental+. In HEALS-Dental+, oral cavity cancer was marginally reduced (p=0.085), and missing teeth was a significant factor for HNC (HR, 1.24; 95% CI, 1.02 to 1.50; p=0.032). Toothbrushing was a significant factor in univariate analysis (p=0.028), but not in multivariate analysis (p=0.877).
Head and neck cancer (HNC) accounts for 1%–2% of all new cases of cancer and cancer-related mortality in Korea [1]. Despite the advances in surgery, radiation, and chemotherapy, which have led to increased survival of patients with HNC in recent decades, the crude 5-year survival rate remains below 70% [1] for all HNC sites combined, warranting better preventive strategies.
There are several known risk factors for the development of HNC, such as smoking, alcohol consumption, and human papillomavirus (HPV) infection [2–5]. Furthermore, the association between poor oral health and risk of HNC has been reported in several observation-al studies [5–9]. Poor oral health factors such as increased tooth loss/defection, few dental visits, poor tooth brushing, or gum disease, are more frequently observed in patients with HNC [6,8]. However, whether improvement of oral health by professional dental examination and education of individuals can contribute to reducing the incidence of HNC remains unknown.
The National Health Insurance System (NHIS), controlled by the Korean government, is the sole health insurance provider in Korea, and covers approximately 97% of Korean citizens. Subjects of the NHIS are encouraged to undergo standardized medical health examinations every 2 years. The NHIS-National Health Screening (NHIS-HEALS) cohort is a nationwide medical examination database, in which biennial standardized health examination is performed for individuals aged 40 years or older [10]. The database includes information on height, weight, laboratory examinations, and a survey on health-related lifestyle factors. A group of subjects in the cohort additionally participated in an oral health screening program (OHSP), including dental examination, education, and recommendations for future oral health care.
In the current study, we evaluated the impact of professional OHSP on reducing the risk of HNC in subjects of the NHIS-HEALS who underwent OHSP compared to those in the NHIS-HEALS cohort who did not undergo oral health examination and education. Furthermore, we investigated whether the individual oral health-related factors at baseline affected the risk of HNC.
The study population in the current study comprised individuals who underwent NHIS-HEALS medical health examination in 2003 and 2004. Follow-up of the cohort continued until the end of 2015. The cohort contained subjects aged 40 years or older with no previous diagnosis of HNC. In this cohort, a group of individuals received additional OHSP by dental professionals (HEALS-Dental+), whereas the remaining subjects underwent routine health check-ups only (HEALS-Dental−). First, HEALS-Dental+ and HEALS-Dental− were compared in terms of the risk of HNC occurrence to evaluate the impact of OHSP. Second, the effect of baseline oral health factors on HNC was evaluated in the HEALS-Dental+ group. Patients with a registered diagnosis of HNC during 2002–2004 from any clinic were excluded to impose a wash-out period.
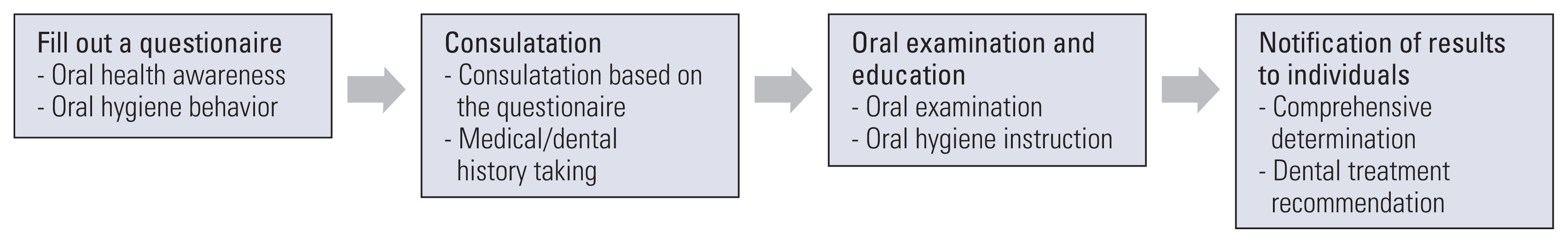
The OHSP protocol begins when the participant visits the screening institution. The participant visits the institution and fills out a questionnaire. Filling out the questionnaire is the process of confirming the oral health awareness and hygiene behaviors of the participant as well as investigating the participant’s medical/dental history. Based on the answers, counseling and oral examination are conducted. Afterwards, the examination process is completed by providing necessary oral hygiene instructions. In case of any dental disease, professional dental care such as caries treatment or periodontal surgery is recommended by dentists. Combining these, the dentist makes a comprehensive decision and informs the patient of the results within 15 days after the OHSP (Fig. 1).
Oral examination by dentists consists of tooth and periodontal tissue examination with a dental mirror and probe. Tooth examination examines conditions related to dental caries as the following: tooth decay or tooth caries (‘yes’ or ‘no’); suspected interproximal tooth caries (‘yes’ or ‘no’); restoration tooth (‘yes’ or ‘no’). Periodontal tissue examination examines missing teeth first. Cases where teeth are lost due to dental caries and cases requiring restoration of function through dental restoration are checked. Afterwards, visual inspection for the presence of gingival inflammation or gingival calculus is performed. Finally, education regarding appropriate oral hygiene care was provided, and in case of any dental disease, professional dental care such as caries control or periodontal surgery was recommended by dentists.
The primary endpoint of the current study was the HNC-free rate after the end of the NHIS-HEALS program. The diagnosis of HNC was confirmed using the International Classification of Diseases, 10th revision (ICD-10) codes for the oral cavity (lip, tongue, gum, floor of mouth, palate, buccal, retromolar trigone), oropharyngeal (base of tongue, lingual tonsil, soft palate/uvula, tonsil, vallecula), hypopharyngeal, laryngeal, nasal cavity/paranasal sinus, and nasopharyngeal cancers (S1 Fig.).
Statistical analyses were performed using the R software (ver. 3.3.3, R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). Univariate and multivariate analyses were performed using the Cox proportional hazards model. Kaplan-Meier curves for the HNC-free rate were generated. The level of statistical significance was set at p < 0.05.
A total of 408,247 subjects without missing data on demographics, socioeconomic status, presence of disability, Charlson comorbidity index, smoking history, and alcohol consumption were included in the study. The HEALS-Dental+ and HEALS-Dental− groups included 165,292 and 242,955 individuals, respectively (S2 Fig.).
During the follow-up period, 1,650 HNC cases (0.40%) were diagnosed. The most prevalent HNC was laryngeal cancer (548/408,247, 0.13%), followed by cancers of the oral cavity, oropharynx, nasopharynx, hypopharynx, and nasal cavity/paranasal sinuses (Table 1). The baseline characteristics of the patients are shown in Table 2. With a median follow-up of 11 years, the 10-year HNC-free rate was 99.684% for all patients.
In univariate analysis, HEALS-Dental+ showed a significantly better HNC-free rate compared to HEALS-Dental− (10-year HNC-free rate, 99.728 vs. 99.654%; p < 0.001) (Table 2, Fig. 2A). Other significant factors for increased risk of HNC included older age, male sex, presence of disability, increased Charlson comorbidity index, smoking history, and alcohol consumption (all p < 0.001) (Table 2). The detrimental impact of HEALS-Dental− on HNC persisted in multivariable analysis (hazard ratio [HR], 1.16; 95% confidence interval [CI], 1.03 to 1.29; p=0.011) (Table 3). To evaluate the favorable impact of HEALS-Dental+ on individual HNC types, we performed further multivariate analyses after adjusting for the same covariables (Table 4). HEALS-Dental+ showed a significantly reduced incidence of oropharyngeal cancer (HR, 1.48; 95% CI, 1.13 to 1.94; p=0.005) (Fig. 2B). Additionally, the risk of oral cavity cancer was marginally reduced in the HEALS-Dental+ group (HR, 1.21; 95% CI, 0.97 to 1.50; p=0.085) (Fig. 2C). In contrast, HEALS-Dental+ did not affect the risk of laryngeal (p=0.543), nasopharyngeal (p=0.156), hypopharyngeal (p=0.426), and nasal cavity/paranasal sinus (p=0.793) cancers.
We further evaluated the impact of each oral health-related factors in the HEALS-Dental+ group (n=165,292). In univariate analysis, the number of missing teeth (p < 0.001) (Fig. 3A) and toothbrushing (p=0.028) (Fig. 3B) were significant oral health-related factors associated with HNC (Table 5). However, the presence of periodontal disease, dental caries, dental visits for any reason, or dental visits for professional cleaning during the previous year did not affect the risk of HNC (Table 5). In multivariate analysis, the presence of missing teeth was the only unfavorable baseline oral health-related factor affecting the risk of HNC (p=0.032) (Table 6).
In the current study, we compared the risk of HNC in recipients (HEALS-Dental+) versus non-recipients (HEALS-Dental−) of professional OHSP among 408,247 subjects. Despite the very low incidence of HNC, HEALS-Dental+ had a significantly decreased risk of HNC by 14% during a 10-year follow-up compared to HEALS-Dental−. Moreover, among the 165,292 individuals in the HEALS-Dental+ group, we observed a significant 25% increase in the risk of HNC in those with missing teeth at baseline. Although toothbrushing was a significant factor in the univariate analysis, the significance diminished after adjusting for other covariates in multivariate analysis.
It has been widely acknowledged that chronic inflammation can lead to cancer development [11]. Periodontitis and poor oral hygiene have been reported to increase the risk of various cancer types, including oral and oropharyngeal cancers [5–9], as well as cancer mortality [12]. Chang et al. [7]reported that lack of regular dental visits, less toothbrushing, gum bleeding, and loss of teeth were positively correlated with HNC. Similarly, Hashim et al. [8] from the International Head and Neck Cancer Epidemiology consortium reported that 12,527 individuals without HNC had significantly fewer missing teeth, regular dental visits, frequent tooth brushing, and absence of gingival disease compared to 8,925 HNC patients. However, in contrast to our study, most of the reports demonstrating a correlation between poor oral health status and HNC development are matched case-control studies involving a single timepoint [5–8]. Therefore, whether oral health status is a modifiable risk factor for HNC could not be definitively determined by these previous case-control series. The significantly reduced risk of HNC in HEALS-Dental+ group observed in our study might be due to several contributions of OHSP (Fig. 4). First, meticulous oral health examination by dentists and management of existing periodontal disease, dental caries, or other morbid conditions may have ceased further inflammatory damage. Second, education concerning appropriate oral health habits such as toothbrushing, use of dental floss, regular dental visits, smoking cessation, or reduction in alcohol consumption might have had a beneficial impact.
The risk reduction associated with OHSP was most notable for oropharyngeal cancer in our study, with the risk reduction exceeding 30%. It is well known that the incidence of HPV-related oropharyngeal squamous cell carcinoma has been increasing worldwide over the past decades, and currently accounts for more than two-third of all oropharyngeal cancers in developed countries [13]. The proportion of HPV-positive oropharyngeal cancers has been reported to be as high 70% [14–16]. Therefore, although our data did not include information on the HPV status, we assumed that the majority of oropharyngeal cancer patients in the current study had HPV infection. Several studies have suggested a correlation between poor oral health, HPV infection, and cancer. Tezal et al. [17] indicated that chronic periodontitis, quantified as mean alveolar bone loss, is correlated with HPV-related cancers of the tongue base. Although Wiener et al. [18] failed to demonstrate a positive correlation between periodontitis and the presence of HPV infection in oral rinse specimens from 6,000 individuals, Sun et al. [19] demonstrated a trend for a positive correlation between HPV infection and poor oral health using the same methodology as Wiener et al. [18]. Furthermore, in a large population-based case-control study, Mazul et al. [20] reported that poor oral health and absence of routine dental visits were significantly more prevalent in both HPV-positive and HPV-negative HNC patients than in controls. The aforementioned hypothetical benefits of OHSP in the HEALS-Dental+ group in terms of oral health might have led to a reduction in oral HPV infection, eventually mitigating the risk of HPV-related oropharyngeal cancers. The risk of oral cavity cancers was also marginally reduced in the HEALS-Dental+ group, whereas the risk of cancers at other subsites was not affected. This observation might be due to the small number of total events, which was inadequate to demonstrate statistical significance compared to other established risk factors such as age, smoking, comorbidities, or alcohol consumption.
To the best of our knowledge, this is the first large-scale nationwide population-based study to evaluate the impact of OHSP on the risk of future HNC development among individuals without a history of HNC. However, the current study has several inherent limitations, such as its retrospective nature and lack of follow-up for oral health conditions, health habits such as cigarette smoking and alcohol intake, and other newly developed comorbidities during the 10-year follow-up period. Nevertheless, not only can the routine administration of OHSP reduce the risk of HNC, improved oral health can also have an impact on occurrence of various diseases such as gastrointestinal cancer, major cardiovascular events, diabetes, and heart disease, as reported by several groups from Korea using the NHIS-HEALS data [21–24].
In summary, the findings of this study strongly support the beneficial impact of a population-based OHSP on reducing the long-term risk of HNC. Significant benefits of OHSP were noted, especially for oropharyngeal cancers. Therefore, routine administration of OHSP as part of NHIS-HEALS should be strongly considered.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was reviewed and approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB No. X-2004/606-905). Patient consent was waived due to the retrospective nature of this study.
Author Contributions
Conceived and designed the analysis: Jeong WJ, Eom KY.
Collected the data: Wee CW, Lee JR.
Contributed data or analysis tools: Wee CW, Lee HJ, Lee H.
Performed the analysis: Lee JR.
Wrote the paper: Wee CW.
Supervision: Lee HJ.
Illustration: Kwoen MJ.
Final approval: Jeong WJ, Eom KY.
References
1. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021; 53:301–15.

2. Bundgaard T, Wildt J, Frydenberg M, Elbrond O, Nielsen JE. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995; 6:57–67.

3. Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biorklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998; 82:1367–75.

4. D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007; 356:1944–56.

5. Marshall JR, Graham S, Haughey BP, Shedd D, O’Shea R, Brasure J, et al. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992; 28B:9–15.

6. Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol. 2005; 125:1327–36.

7. Chang JS, Lo HI, Wong TY, Huang CC, Lee WT, Tsai ST, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013; 49:1010–7.

8. Hashim D, Sartori S, Brennan P, Curado MP, Wunsch-Filho V, Divaris K, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol. 2016; 27:1619–25.

9. Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One. 2018; 13:e0195683.

10. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017; 7:e016640.

11. Cao X, Xu J. Insights into inflammasome and its research advances in cancer. Tumori. 2019; 105:456–64.

12. Heikkila P, But A, Sorsa T, Haukka J. Periodontitis and cancer mortality: register-based cohort study of 68,273 adults in 10-year follow-up. Int J Cancer. 2018; 142:2244–53.

13. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29:4294–301.

14. Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007; 120:1418–25.

15. Kim Y, Joo YH, Kim MS, Lee YS. Prevalence of high-risk human papillomavirus and its genotype distribution in head and neck squamous cell carcinomas. J Pathol Transl Med. 2020; 54:411–8.

16. Choi I, Lee D, Son KB, Bae S. Incidence, cost and gender differences of oropharyngeal and noncervical anogenital cancers in South Korea. BMC Public Health. 2020; 20:1035.

17. Tezal M, Sullivan Nasca M, Stoler DL, Melendy T, Hyland A, Smaldino PJ, et al. Chronic periodontitis-human papilloma-virus synergy in base of tongue cancers. Arch Otolaryngol Head Neck Surg. 2009; 135:391–6.

18. Wiener RC, Sambamoorthi U, Jurevic RJ. Association of periodontitis and human papillomavirus in oral rinse specimens: results from the National Health and Nutrition Survey 2009–2012. J Am Dent Assoc. 2015; 146:382–9.
19. Sun CX, Bennett N, Tran P, Tang KD, Lim Y, Frazer I, et al. A pilot study into the association between oral health status and human papillomavirus-16 infection. Diagnostics (Basel). 2017; 7:11.

20. Mazul AL, Taylor JM, Divaris K, Weissler MC, Brennan P, Anantharaman D, et al. Oral health and human papilloma-virus-associated head and neck squamous cell carcinoma. Cancer. 2017; 123:71–80.

21. Lee K, Lee JS, Kim J, Lee H, Chang Y, Woo HG, et al. Oral health and gastrointestinal cancer: a nationwide cohort study. J Clin Periodontol. 2020; 47:796–808.

22. Park SY, Kim SH, Kang SH, Yoon CH, Lee HJ, Yun PY, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J. 2019; 40:1138–45.

Fig. 2
Kaplan-Meier curves of cancer-free rates for head and neck cancers (A), oropharyngeal cancers (B), and oral cavity cancers (C) depending on the receipt of national oral health screening program.
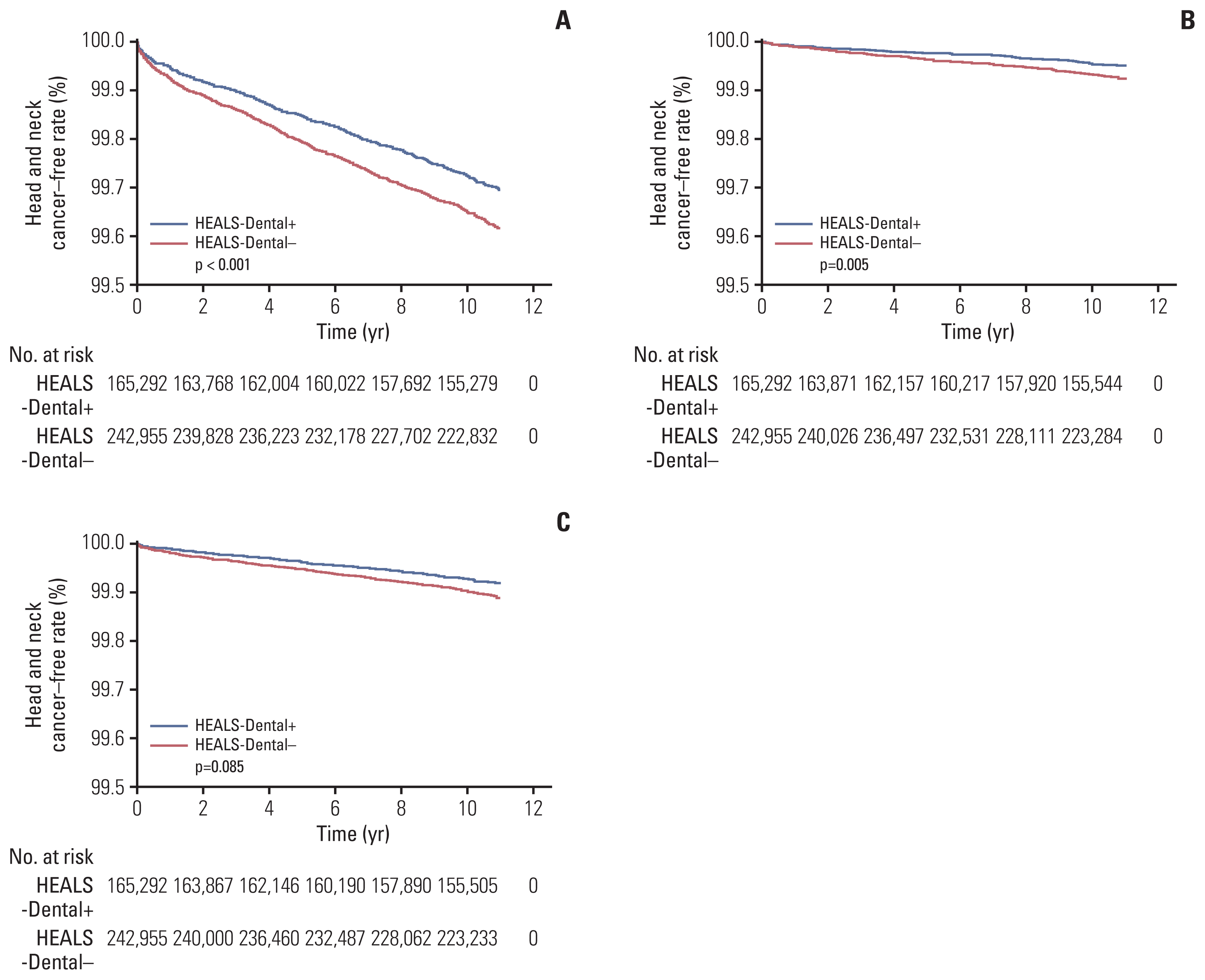
Fig. 3
Kaplan-Meier curves of head and neck cancer–free rates depending on number of missing teeth (A) and toothbrushing in individuals receiving the national oral health screening program (B).
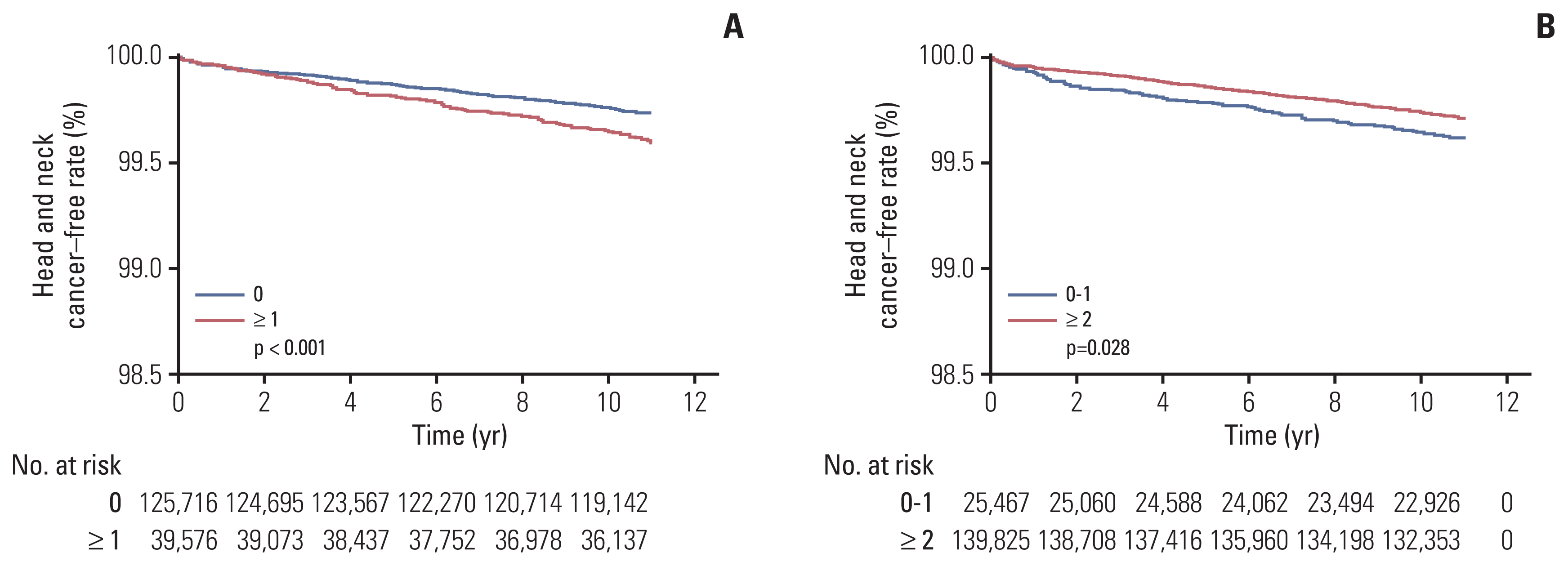
Fig. 4
Summary of the proposed effect of national oral health screening program in reducing head and neck cancer. HPV, human papillomavirus.
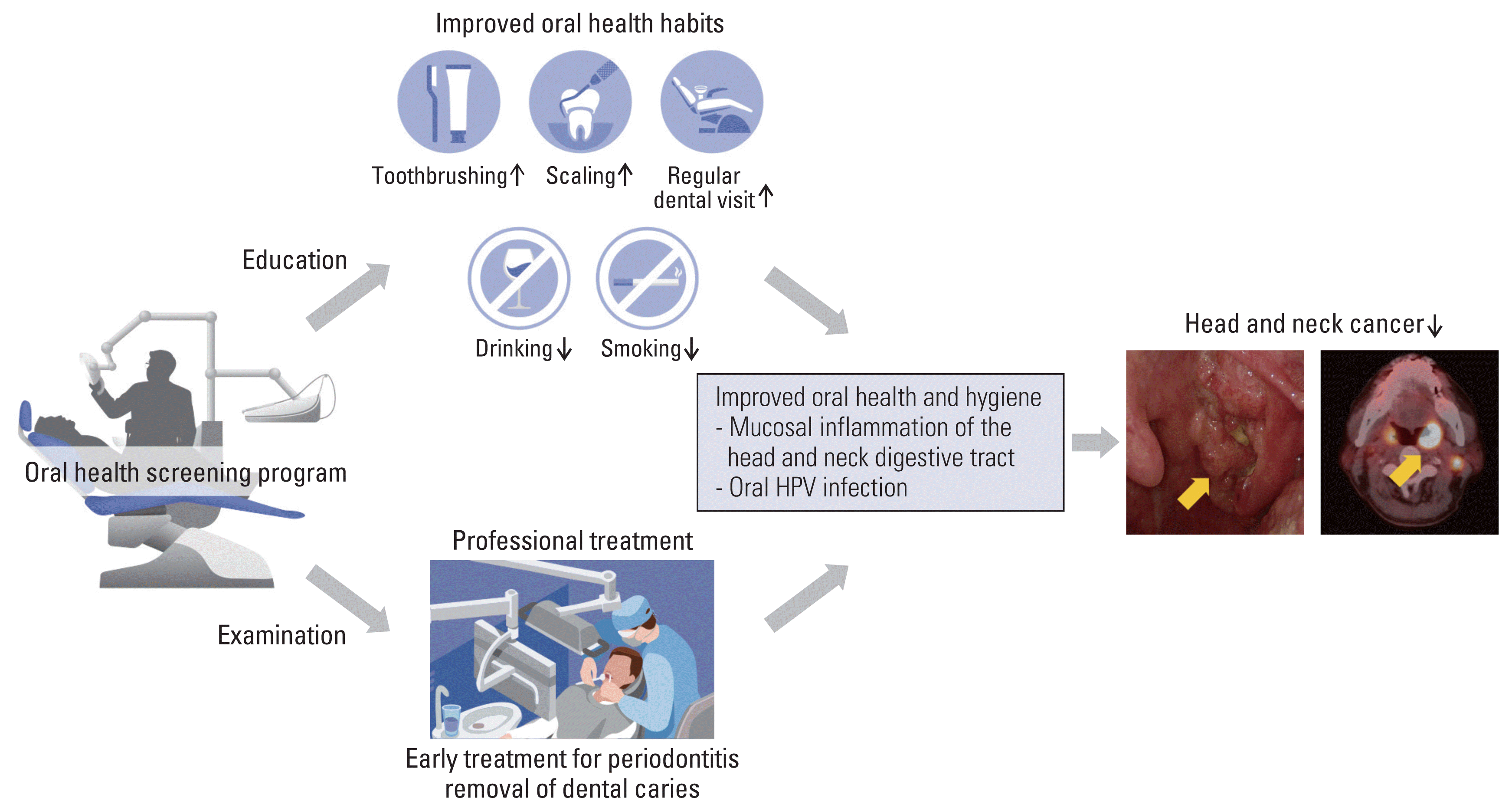
Table 1
Incidence of head and neck cancer by site in all patients
| Subsite | No. (%) (n=408,247) |
|---|---|
| Oral cavity | 382 (0.09) |
| Oropharynx | 253 (0.06) |
| Hypopharynx | 155 (0.04) |
| Larynx | 548 (0.13) |
| Nasal cavity/Paranasal sinus | 142 (0.03) |
| Nasopharynx | 170 (0.04) |
Table 2
Baseline characteristics of the study population and univariate analysis for the risk of head and neck cancer
Table 3
Multivariate analysis for the risk of head and neck cancer
Table 4
Multivariate analysis on increased risk of each head and neck cancer type by not receiving oral health screening
| Cancer subsite | HRa) (95% CI) | p-valueb) |
|---|---|---|
| Oral cavity | 1.21 (0.97–1.50) | 0.085 |
| Oropharynx | 1.48 (1.13–1.94) | 0.005 |
| Hypopharynx | 1.15 (0.82–1.61) | 0.426 |
| Larynx | 1.06 (0.89–1.26) | 0.543 |
| Nasal cavity/Paranasal sinus | 0.96 (0.68–1.34) | 0.793 |
| Nasopharynx | 1.26 (0.92–1.74) | 0.156 |
Table 5
Baseline characteristics of the population receiving oral health screening (HEALS-Dental+) and univariate analysis for risk of head and neck cancer
Table 6
Multivariate analysis of oral health-related factors for the risk of head and neck cancer




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download