Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
The Institutional Review Board of Samsung Medical Centre approved this study (IRB No. 2018-03-062-002) and waived the requirement for informed consent due to its retrospective nature.
Author Contributions
Conceived and designed the analysis: Um SW.
Collected the data: Kang N, Kim KH, Jeong BH, Lee K, Kim H, Kwon OJ, Ahn MJ, Cho J, Lee HY, Um SW.
Contributed data or analysis tools: Kang N, Kim KH, Jeong BH, Lee K, Kim H, Kwon OJ, Ahn MJ, Cho J, Lee HY, Um SW.
Performed the analysis: Kang N, Kim KH, Lee HY, Um SW.
Wrote the paper: Kang N, Lee HY, Um SW.
Acknowledgments
References
Fig. 1
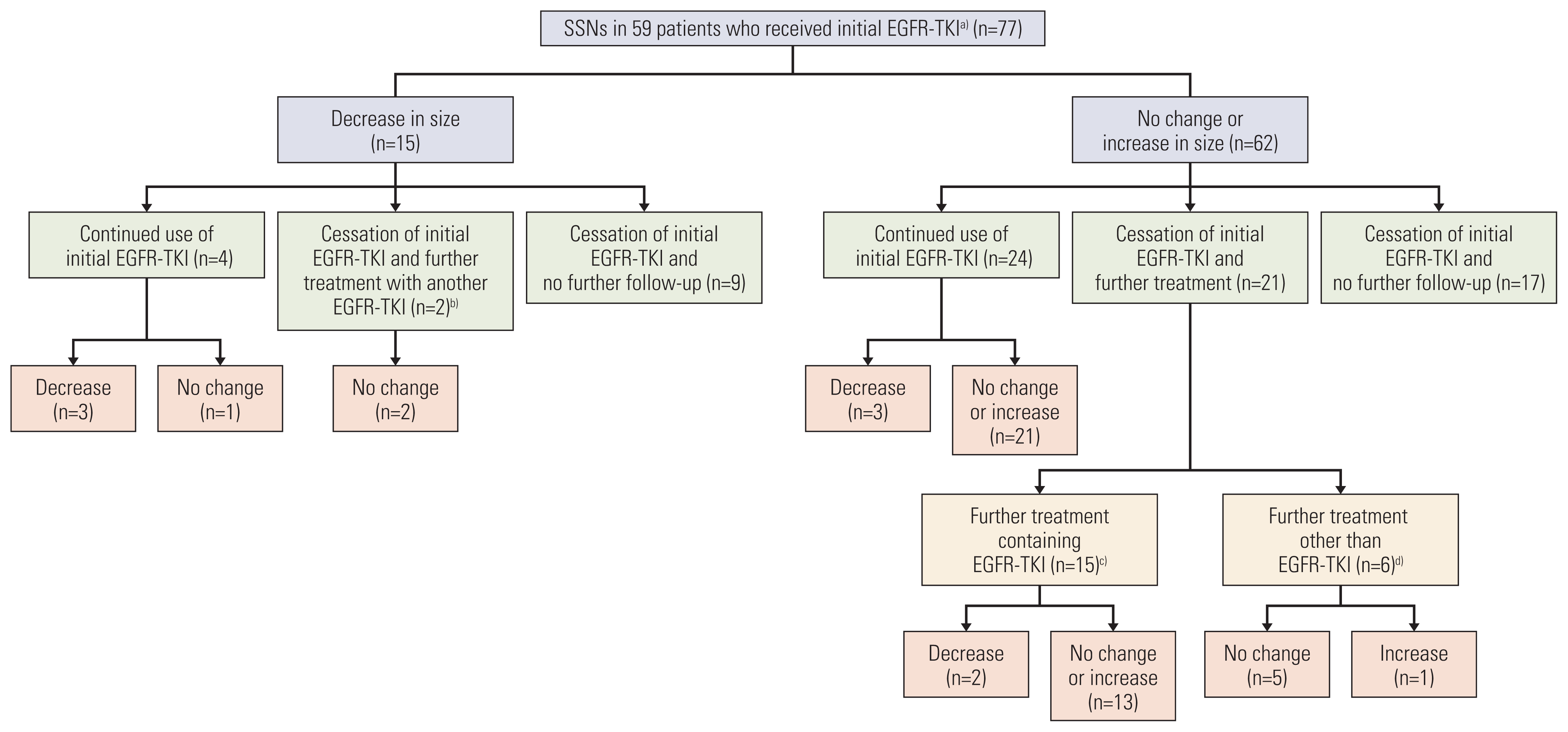
Fig. 2
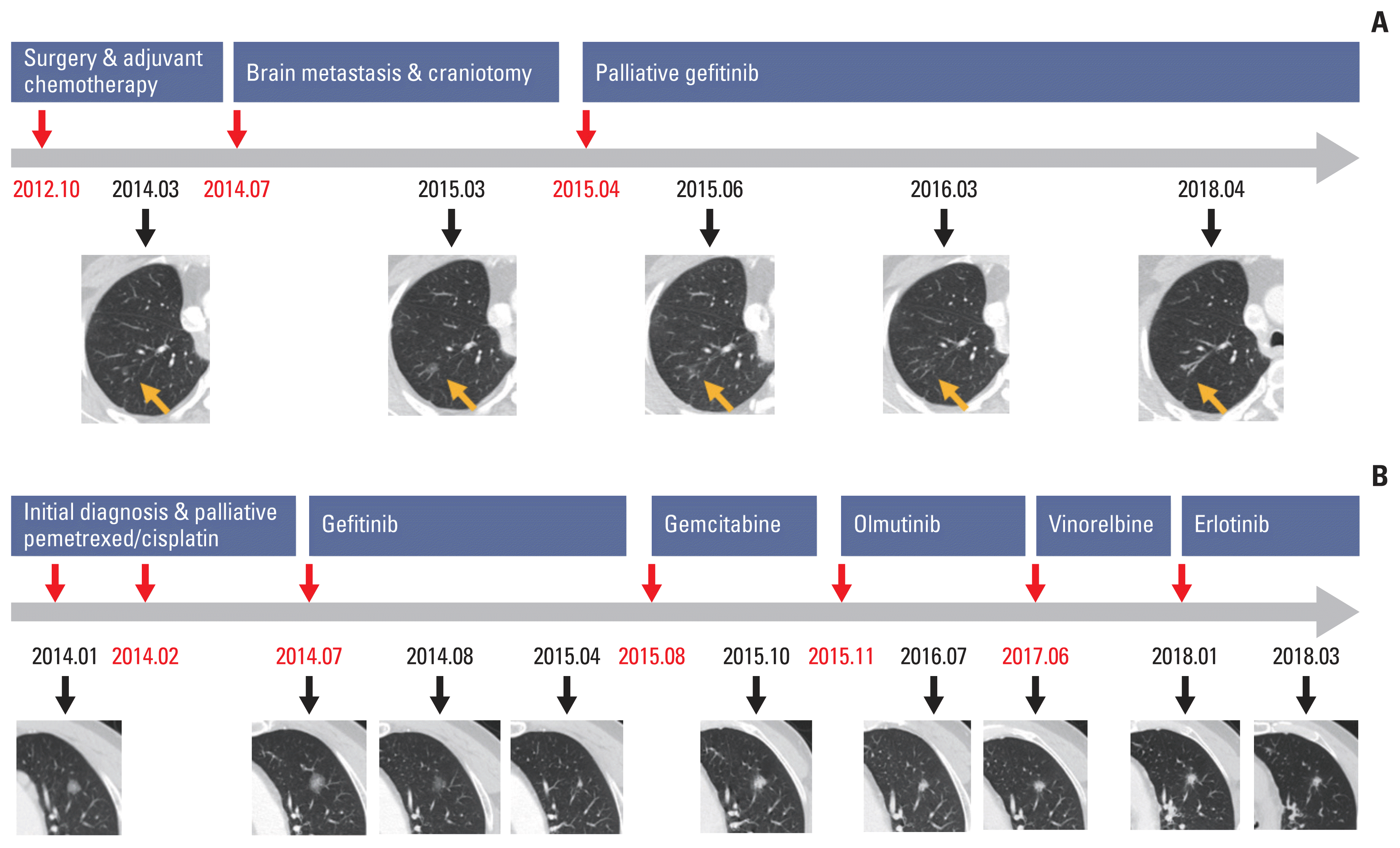
Table 1
| Characteristic | Total | Decrease (n=15)a) | No change or increase (n=44)b) | p-value |
|---|---|---|---|---|
| Age (yr) | 58 (54–67) | 58 (55–66) | 58 (53–67) | 0.940 |
| Sex | ||||
| Male | 20 (33.9) | 5 (33.3) | 15 (33.3) | 0.957 |
| Female | 39 (66.1) | 10 (66.7) | 29 (65.9) | |
| Smoking history | ||||
| Never-smoker | 44 (74.6) | > 0.99 | ||
| Former or current smoker | 15 (25.4) | 4 (26.7) | 11 (25.0) | |
| History of other malignancy | 9 (15.3) | 4 (26.7) | 5 (11.4) | 0.213 |
| Previous lung cancer treatment modalityc) | ||||
| Surgical resection | 26 (44.1) | 6 (40.0) | 20 (45.5) | 0.713 |
| Radiotherapy (to lung) | 13 (22.0) | 3 (20.0) | 10 (22.7) | > 0.99 |
| Neoadjuvant chemotherapy/CCRT | 4 (6.8) | 0 | 4 (9.1) | 0.564 |
| Definitive CCRT | 4 (6.8) | 0 | 4 (9.1) | 0.564 |
| Adjuvant chemotherapy | 15 (25.4) | 3 (20.0) | 12 (27.3) | 0.738 |
| Palliative chemotherapy | 32 (54.2) | 9 (60.0) | 23 (52.3) | 0.604 |
| EGFR-TKI as first-line chemotherapy | 9 (15.3) | 3 (20.0) | 6 (13.6) | 0.680 |
| Type of EGFR mutation for primary lung cancer (n=58)d) | ||||
| EGFR wild type | 13/58 (22.4) | 2 (14.3) | 11 (25.0) | 0.489 |
| EGFR mutation (+) | 45/58 (77.6) | 12 (85.7) | 33 (75.0) | 0.031 |
| Deletion in exon 19 | 22/58 (37.9) | 9 (75.0) | 13 (39.4) | |
| Missense mutation in exon 21 (L858R) | 17/58 (29.3) | 1 (8.3) | 16 (48.5) | |
| Uncommon mutatione) | 6/58 (10.3) | 2 (16.7) | 4 (12.1) | |
| Site where EGFR mutation was detected (n=45) | ||||
| Primary lesion (lung parenchyma or airway) | 35/45 (77.8) | 10 (83.3) | 25 (75.8) | 0.845 |
| Metastatic lymph nodes | 7/45 (15.6) | 2 (16.7) | 5 (15.2) | |
| Other metastatic lesionsf) | 3/45 (6.7) | 0 | 3 (9.1) | |
| Regimen of initial EGFR-TKI | ||||
| Gefitinib | 45 (76.3) | 10 (66.7) | 35 (79.5) | |
| Erlotinib | 14 (23.7) | 5 (33.3) | 9 (20.5) | |
| Best response of initial EGFR-TKI for primary lung cancer | ||||
| Partial remission | 37 (62.7) | 9 (60.0) | 28 (63.6) | 0.692 |
| Stable disease | 11 (18.6) | 2 (13.3) | 9 (20.5) | |
| Progressive disease | 11 (18.6) | 4 (26.7) | 7 (15.9) | |
| Type of SSNg) | ||||
| pGGN | 35 (59.3) | 8 (53.3) | 27 (61.4) | 0.762 |
| PSN | 24 (40.7) | 7 (46.7) | 17 (38.6) | |
| From SSN detection to initiation of initial EGFR-TKI (day) | 307 (182–583) | 215 (159–688) | 336 (193–555) | 0.645 |
| Duration of initial EGFR-TKI (day) | 308 (97–583) | 357 (56–623) | 270 (104–534) | 0.974 |
| From initial EGFR-TKI discontinuation to last follow-up (day) (n=51) | 693 (308–1,132) | 624 (293–1,172) | 718 (308–1,123) | 0.860 |
Values are presented as median (interquartile range) or number (%). CCRT, concurrent chemoradiation therapy; EGFR, epidermal growth factor receptor; pGGN, pure ground-glass opacity nodule; PSN, part-solid nodule; SSN, subsolid nodule; TKI, tyrosine kinase inhibitor.
a) Decrease (size change ≤ −2 mm) (n=10) or mixed response which had multiple lesions with both decrease and no change (−2 mm < size change < +2 mm) (n=5),
Table 2
Table 3
| Before initial EGFR-TKI (mm) | After initial EGFR-TKI (mm) | p-value | |
|---|---|---|---|
| Total (n=77) | 7.1 (6.0–9.7) | 6.8 (5.2–8.9) | 0.001 |
| pGGN (n=51) | 6.6 (5.9–8.7) | 6.5 (4.8–8.0) | < 0.001 |
| PSN (n=26) | 8.8 (7.0–12.3) | 8.3 (6.6–10.3) | 0.182 |
| EGFR mutation (+) (n=59) | 7.1 (5.9–9.6) | 6.6 (4.8–8.9) | < 0.001 |
| Deletion in exon 19 (n=26) | 8.2 (6.2–12.1) | 6.6 (4.8–8.7) | < 0.001 |
| Missense mutation in exon 21 (L858R) (n=23) | 7.0 (5.6–8.9) | 6.8 (4.6–8.9) | 0.048 |
| Uncommon mutation (n=10)b) | 6.4 (5.4–9.4) | 6.0 (5.0–8.8) | 0.097 |
| EGFR wild type (n=17) | 7.5 (6.0–10.5) | 7.7 (6.6–9.0) | 0.587 |
Values are presented as median (interquartile range). EGFR, epidermal growth factor receptor; pGGN, pure ground-glass nodule; PSN, part-solid nodule; SSN, subsolid nodule; TKI, tyrosine kinase inhibitor.
Table 4
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (yr) | 1.00 (0.94–1.07) | 0.939 | - | - |
|
|
||||
| Female sex (vs. male) | 1.03 (0.30–3.58) | 0.967 | - | - |
|
|
||||
| Ever-smoked (vs. never smoked) | 1.09 (0.29–4.14) | 0.898 | - | - |
|
|
||||
| History of other malignancy (vs. no history) | 2.84 (0.65–12.40) | 0.166 | - | - |
|
|
||||
| Part-solid nodule (vs. pure GGN) | 1.53 (0.47–5.01) | 0.481 | - | - |
|
|
||||
| Initial EGFR-TKI as first-line chemotherapy (vs. second-line ormore) | 1.58 (0.34–7.32) | 0.556 | - | - |
|
|
||||
| Gefitinib (vs. erlotinib) | 1.94 (0.53–7.13) | 0.316 | - | - |
|
|
||||
| EGFR exon 19 del positivity for primary lung cancer (vs. all others)a) | 3.58 (1.06–12.11) | 0.040 | 4.29 (1.21–15.29) | 0.025 |
|
|
||||
| Best response of initial EGFR-TKI for primary lung cancer (partial remission vs. stable disease/progressive disease) | 0.86 (0.26–2.85) | 0.802 | - | - |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download