Abstract
Purpose
The clinical significance of body mass index (BMI) on long-term outcomes has not been extensively investigated in Asian patients with colorectal cancer (CRC). This study aims to describe the association between BMI and survival, plus providing BMI cutoff value for predicting prognosis in CRC patients.
Materials and Methods
A total of 1,182 patients who had undergone surgery for stage I–III CRC from June 2004 to February 2014 were included. BMI was categorized into four groups based on the recommendation for Asian ethnicity. The optimal BMI cutoff value was determined to maximize overall survival (OS) difference.
Results
In multivariable analysis, underweight BMI was significantly associated with poor OS (hazard ratio [HR], 2.38; 95% confidence interval [CI], 1.55 to 3.71; p < 0.001) and obese BMI was associated with better OS (HR, 0.72; 95% CI, 0.53 to 0.97; p=0.036) compared with the normal BMI. Overweight and obese BMI were associated with better recurrence-free survival (HR, 0.64; 95% CI, 0.42 to 0.99; p=0.046 and HR, 0.58; 95% CI, 0.38 to 0.89; p=0.014, respectively) compared with the normal BMI group. BMI cutoff value was 20.44 kg/m2. Adding the BMI cutoff value to cancer staging could increase discriminatory performance in terms of integrated area under the curve and Harrell’s concordance index.
Obesity is a well-known risk factor of colorectal cancer (CRC) [1–3]. Proinflammatory factors, immune suppression, and changes in the metabolic functions of adipose tissue play an active role in establishing and promoting tumoral progression [1,4]. For this reason, many researchers have investigated the fundamental association of obesity with prognosis [5–13], however, the relationship is yet to be elucidated in patients with CRC. Although it is widely accepted that people with obesity are prone to cancer [14], many report that patients with cancer and overweight live longer [7,10,12], which is a phenomenon called the ‘obesity paradox’. The reason for this remains a controversial issue. Some studies suggest that methodological errors, such as selection bias, reverse causality, and collider bias could explain this paradox [3,10].
Furthermore, the distribution of body weight significantly varies among races. Asians tend to store their body fat viscerally rather than subcutaneously [15–17]. Body mass indexes (BMIs) of Asians tend to be lower than those of Caucasians, and even at the same BMI, Asians experience less favorable prognoses than Caucasians [16,17]. To account for this discrepancy, the World Health Organization (WHO) set different BMI cutoff values for obesity [15]. However, since previous research has focused primarily on Caucasians, further evidence regarding the obesity paradox in Asians is needed. Regarding Asians, the obesity paradox was reported less frequently. Although previous studies have suggested BMI cutoff values that predict survival of CRC patients [4,18], more data is needed as controversy remains on the existence and mechanism of obesity paradox.
We therefore analyzed the association between BMI and two main outcomes, overall survival (OS) and recurrence-free survival (RFS) among Korean patients. We identified the optimal BMI cutoff that effectively discriminates survival and tested the clinical significance of this index.
Patients who underwent curative resection of stage I–III CRCs at Gangnam Severance Hospital, Yonsei University College of Medicine between June, 2004 and February 2014 were enrolled in this study. Patients who had unusual histopathological types (neuroendocrine or gastrointestinal stromal tumor), tumors in certain locations (appendix or anus), hereditary colon cancer, Crohn’s disease, or primary cancer in multiple sites were excluded. Patients who received preoperative chemotherapy, chemoradiotherapy, or an emergency operation were also excluded. We acquired a total of 1,182 patients for our study (S1 Fig.).
Preoperative staging included a physical examination, plain radiology, colonoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), as appropriate. All resections were performed according to a standardized technique, and were either total mesorectal excisions or complete mesocolic excisions. Curative resection was defined as the absence of any gross residual tumor and a surgical resection margin that was pathologically negative for tumor invasion. Postoperative chemotherapy was mainly recommended for high-risk patients with stage II and stage III CRC.
Patients were followed up, using our standard protocol, every 3–6 months for at least 5 years. This protocol included tumor marker tests, CT, and chest radiography. Bone scans were performed when bone metastasis was indicated. Colonoscopy, pelvic MRI, and PET scan were selectively carried out at the physician’s discretion.
Data collected from inpatient and outpatient records included demographic data (age, sex, height, weight), tumor-specific data (pathological data) including T classification, lymph node metastasis, differentiation, lymphovascular invasion (LVI), tumor marker data including carcinoembryonic antigen (CEA) levels at diagnosis, recurrence, and survival outcomes.
BMI was calculated by dividing the patient’s weight in kilograms by the patient’s height in meters squared. The height and weight data used to calculate BMI were measured at the time of surgery. Patients were grouped into four BMI categories according to the WHO definition of Asian-specific values [15]: underweight, < 18.5 kg/m2; normal, 18.5–22.9 kg/m2; overweight, 23–24.9 kg/m2; and obese, ≥ 25 kg/m2.
Categorical variables and continuous variables were analyzed by the chi-square test and analysis of variance (ANOVA) test, respectively. OS was defined as time from date of surgery to date of death from any cause or date of last follow up. RFS was defined from the date of surgery to the date of detection of recurrence or last follow-up or death. Comparison of OS or RFS across BMI categories was performed using Kaplan-Meier curves and the log-rank test. Crude and adjusted hazard ratios (HRs) and corresponding 95% confidence interval (CI) were computed using Cox-proportional hazards modeling. Variables that had significance of p < 0.05 on univariable analysis were eligible for inclusion in multivariable analysis. Factors associated with OS or RFS were analyzed by a backward stepwise selection of variables in the multivariable analysis.
We visualized the change of hazard rate of OS according to BMI using restricted cubic splines. We used model parsimony and the Akaike information criterion for choosing the optimal number of knots in the model.
Next, the X-tile program [19] was used to determine the optimal cutoff value of BMI that effectively discriminates OS and RFS. For the validation of our newly developed BMI cutoff value, we divided our study population (n=1,182) into two groups, training set (n=827) and test set (n=355) by random sampling. We tried to define new cutoff value using the training set and validated the newly defined value in the test set, Also, in the training set, we have tested if a three-tiered cutoff value would be useful in prediction of OS.
The discriminatory accuracy was compared between stage plus BMI cutoff value and stage only in the test set, by calculating the integrated area under the curve (iAUC) and Harrell’s concordance index (C-index). The iAUC determines the relevance of a certain biomarker in the disease status, as it takes both the changing levels of the biomarker and disease status throughout time into account. It is derived by integrating the time-dependent receiver operating characteristic curve of the selected biomarker over time. The C-index estimates the discriminating ability of the survival-predicting model. A C-index close to 1 denotes that the model accurately predicts one’s survival [20]. We calculated the C-index by a bootstrapping method.
All statistical analyses were performed using R ver. 3.6.3 (R-project, Institute for Statistics and Mathematics, Vienna, Austria). A two-sided p < 0.05 was considered statistically significant.
Of the 1,182 patients, 476 (40.3%) were female and 706 (59.7%) were male. The majority of the patients were 60 or more years old (n=744, 62.9%). The median follow-up period was 95 months (interquartile range, 75 to 127 months). A total of 277 patients (23.4%) died and 144 patients (12.2%) showed recurrence during the study period.
Patients were categorized as underweight (n=61, 5.2%), normal (n=450, 38.1%), overweight (n=317, 26.8%) and obese (n=354, 29.9%) according to the WHO definition of Asian-specific BMI values. Differences in BMI categories were observed for hypertension history rate (p < 0.001), tumor size (p=0.007), histologic grade (p=0.021), presence of LVI (p=0.043), and more than 12 total retrieved lymph nodes (p=0.017). The groups were balanced with respect to sex, age, diabetes mellitus (DM) history, preoperative CEA, tumor location, tumor stage, complications, and receipt of chemotherapy (Table 1).
The Kaplan-Meier curve for OS showed a significant difference in survival according to BMI category (5-year OS: underweight, 68.5%; normal, 83.5%; overweight, 85.6%; and obese, 89.8%; normal vs. underweight, p < 0.001; normal vs. overweight, p=0.626; normal vs. obese, p=0.080) (Fig. 1A). The Kaplan-Meier curve for RFS showed a significant difference for recurrence according to BMI category (5-year RFS: underweight, 78.8%; normal, 84.4%; overweight, 89.7%; and obese, 91.6%; normal vs. underweight, p=0.225; normal vs. overweight, p=0.019; normal vs. obese, p=0.003) (Fig. 1B).
Table 2 shows the results of the univariable and multivariable Cox regression analyses for OS. In the univariable analysis, sex, age, DM, BMI, hypertension, CEA, tumor size, postoperative complications, LVI, retrieved lymph nodes, and stage were significantly associated with OS. Multivariable analysis showed that older age, underweight and obese status, high CEA, large tumor size, postoperative complications, less retrieved lymph nodes, and advanced stage were independently related with poorer survival. In particular, apart from well-known variables that determine survival such as CEA, tumor size, and stage in patients with CRC, being underweight affected survival with an HR of 2.27 (95% CI, 1.49 to 3.46). In contrast, obesity had a protective effect on survival, with an HR of 0.72 (95% CI, 0.35 to 0.97).
Factors significantly associated with RFS were BMI (overweight and obesity), high CEA, postoperative complications, histologic grade, LVI, stage, and receipt of chemotherapy. Multivariable analysis showed that high CEA, LVI, and advanced stage increased the HR of cancer recurrence. Conversely, having overweight and obesity had a protective effect against recurrence, in comparison with the normal BMI group (overweight: HR, 0.64; 95% CI, 0.42 to 0.99, p=0.046; obese: HR, 0.58; 95% CI, 0.38 to 0.89; p=0.014). Being underweight was not associated with RFS (HR, 1.38; 95% CI, 0.74 to 2.55), in contrast to OS (Table 3).
Restricted cubic splines were plotted to visualize the effect of BMI on OS and RFS. BMI was prognostic of OS and RFS in a reverse J-shaped curve, as shown in Fig. 2. Regarding OS, risks were higher in people with underweight, and female patients with overweight experienced a longer lifespan unlike male patients. Comparison of RFS according to BMI showed that underweight was associated with more recurrence, while overweight or obesity was not.
In the training set (n=827), using the X-tile program, we found that 20.44 is the optimal BMI cutoff value that effectively discriminates OS (S2 Fig.). In the test set (n=355), Kaplan-Meier curves representing OS and RFS were drawn after stratification by this cutoff value. OS was significantly higher in the BMI ≥ 20.44 group than that in the BMI < 20.44 group (5-year OS: BMI ≥ 20.44, 89.1% vs. BMI < 20.44, 76.0%; p < 0.001). RFS was significantly higher in the BMI ≥ 20.44 group than that in the BMI < 20.44 group (5-year RFS: BMI ≥ 20.44, 91.9% vs. BMI < 20.44, 81.1%; p=0.004) (Fig. 3). We also found that the optimal cutoff of BMI according to the RFS was 20.71 kg/m2 (S3 Fig.), which is similar to the cutoff defined by OS.
We tried to define three-tier classification in the training set using X-tile program. The suggested cutoff values of three tier classification were 20.44 kg/m2 and 24.73 kg/m2. The BMI < 20.44 group showed the worst prognosis compared to the other two groups, which is in line with the result shown in the two-tiered approach. Nevertheless, it seems that survival outcome is similar between the two groups with higher BMI (S4 Fig.). Thus, we believe that three-tier classification has no additive clinical significance compared to the two-stage classification.
We evaluated the clinical implication of the newly developed BMI cutoff value (20.44) in the test set. The iAUC value of stage plus BMI cutoff for OS (0.658; 95% CI, 0.600 to 0.712) was superior to that of stage (0.611; 95% CI, 0.549 to 0.668; bootstrap iAUC mean difference, 0.047; 95% CI, 0.015 to 0.089) throughout the observation period. The iAUC value of stage plus BMI cutoff value for RFS (0.668; 95% CI, 0.588 to 0.750) was superior to that of stage (0.624; 95% CI, 0.540 to 0.705; bootstrap iAUC mean difference, 0.043; 95% CI, 0.004 to 0.106) throughout the observation period (Fig. 4).
The C-index of stage plus BMI cutoff value showed superior discriminatory power compared with stage alone for OS (0.680 vs. 0.636; estimated difference, 0.044) and RFS (0.675 vs. 0.634; estimated difference, 0.041), respectively (Table 4).
This study demonstrated that BMI, stratified by Asian-specific categories, is an independent prognostic factor for OS and RFS respectively, after adjusting for possible confounders. Being underweight was associated with shorter survival, whereas overweight or obesity was related with longer survival compared with normal BMI. A BMI cutoff value of 20.44 kg/m2 was set as an effective criterion for dichotomization, which maximizes the capability of predicting OS. Combining this BMI cutoff value with stage showed clinically added value for discriminating prognosis compared with stage alone, as assessed by comparing the iAUC and C-index.
The association between underweight BMI and poor survival is coherently observed in patients with CRC [4,7,8, 10,18,21,22]. However, one study showed that underweight BMI showed no significant difference in OS compared to normal BMI in patients with stage III CRC (HR, 1.29; 95% CI, 0.77 to 2.19) [5], although the exact reason of this discordance was unclear. Some speculate that being underweight reflects a more advanced disease status and this may be translated into worse outcomes [8,23]. In our study, the rate of stage III also tended to be higher in patients with underweight BMI, although this did not reach statistical significance. In addition, common sarcopenic status in patients with underweight BMI may account for considerable association with poor oncologic outcomes [24,25]. Various inflammatory substances such as tumor necrosis factor α (TNF-α), interleukin (IL)-1α, and IL-6 are prevalent in patients with cachexia, and TNF-α can stimulate tumor formation by inducing insulin resistance [14,24]. Nutrients from gluconeogenesis or amino acids produced by muscle wasting are increased in patients with cancer cachexia, and this may cause tumors to grow. This suggests malnourishment and cachexia with a lack of physiological reserves, which were more common in patients with under-weight BMI, seem to play a role. Our data revealed that underweight BMI was associated with poor OS compared with patients with normal BMI, whereas there was no significant difference in RFS. Therefore, the underlying cause of poor prognosis in underweight patient needs clarification.
Whether overweight is associated with better survival remains a debatable topic and studies regarding patients with CRC have offered conflicting insights: some report being overweight has a protective effect [7,10,12], some report a hazardous effect [21], and some failed to find an association at all [5,6]. In terms of obesity and survival, the better survival outcome in patients with overweight is known as the ‘obesity paradox.’ This relation is observed in many cancer types, including colorectal, renal, hematologic, and metastatic malignancies [26]. There are possible explanations for this phenomenon. Higher BMI reflecting a higher reserve of protein and energy for healing and receiving chemotherapy may be the underlying biological mechanism [3,27]. Methodological errors such as selection bias, reverse causality, and collider bias are also assumed to be the cause [26,28], although a study designed to minimize the biases found that the paradox is conserved even after correction [10]. Ujvari et al. [29] suggested that the immune system, which fights indolent types of precancerous colonies before they differentiate into cancer works more favorable in normal-weight people than obese people. Therefore, the proportion—not the absolute number—of aggressive cancers may be lower in cancer patients with obesity than normal weight, leading to a seemingly better survival in the former [29]. Although this is an interesting explanation, further clinical and experimental data supporting this hypothesis are needed.
We found that obesity was associated with better OS, and overweight and obesity had a protective effect against cancer recurrence. Our finding supports the existence of the obesity paradox regarding OS and RFS, even in Asian patients with CRC. Nonetheless, a large body of literature demonstrates that patients with overweight and obesity have higher rates of CRC recurrence [2,6,30]. A brief review of current literature regarding the association between BMI and CRC is shown in Table 5.
Our study was unable to explain why the association of obesity with survival differs between studies, although the explanation is likely multifactorial. The different effect of fat distribution and their adverse effects among races may be the main reason [15,17].
Because BMI is closely related to survival outcomes, we evaluated the clinical utility of BMI on predicting prognosis in patients with non-metastatic CRC. A cutoff value of 20.44 kg/m2 was proven to be an effective criterion for this purpose. Furthermore, aside from cancer stage, which is the current standard for predicting prognosis, we showed that BMI is also a useful and convenient prognosticator when combined with cancer stage. Its added value was assessed using iAUC and C-index. This cutoff value is similar to that of 20.2 kg/m2 used by a study based on a huge Korean population [18]. A Japanese study also showed that BMI 20 kg/m2 is a significant dichotomization value for predicting OS and RFS [4]. In this study, it was confirmed once again that a BMI reference value similar to the reference value proposed in two previous Asian studies could accurately predict a patient’s prognosis. In particular, it was confirmed that when the patient’s prognosis was predicted using the combination of BMI cutoff and staging, the prognosis could be predicted more accurately than through the cancer staging. This could confirm in more detail that BMI can be helpful in predicting the prognosis of patients. However, the clinical usefulness of this cutoff has mainly been validated in Asian patients with CRC. Considering that poor prognosis has also been reported in underweight BMI in most of the previous studies, its clinical effect needs to be assessed in western patients with CRC.
Our study had some limitations. Only BMI was used for classification of obesity, and this index does not reflect the visceral or subcutaneous distribution of fat. The relatively small subgroup sizes, especially for underweight BMI, could have possibly reduced the validity our results. Socioeconomic data, menopausal status, and diet were also not considered. Most of all, our mean BMI was around 23 kg/m2 and only approximately 2.2% of patients had a BMI of more than 30 kg/m2. The small sample size of this group did not allow for the analysis of BMI categorization of class I–III obesity. This difference of BMI distribution makes it difficult for our results to be generalized. Nevertheless, our study boasts outstanding strengths. The main forte of this study is that we quantitively assessed the significance of BMI cutoff value when incorporated into clinical decision-making. Other strengths include a long follow-up period, visualization using restricted cubic splines, and a large sized cohort of the Asian population.
In conclusion, BMI was prognostic of OS and RFS for patients with stage I–III CRC. The results support the existence of the obesity paradox. The strength of this relationship varies by patient sex. We determined a BMI cutoff value of 20.44 kg/m2 for discriminating the prognosis of patients with CRC, and the clinical effectiveness of the index was demonstrated. BMI could be used to improve prognostic discrimination and should be integrated into clinical decision-making.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board of our institute. The requirement for informed consent was waived for this retrospective study (IRB No. 3-2020-0443).
Author Contributions
Conceived and designed the analysis: Kang J.
Collected the data: Lee DH, Shin SJ, Park EJ, Baik SH, Lee KY, Kang J.
Contributed data or analysis tools: Lee S, Lee JH, Lee HS, Kang J.
Performed the analysis: Lee S, Lee JH, Lee HS, Kang J.
Wrote the paper: Lee S, Kang J.
Critical revision of the manuscript for important intellectual content: Shin SJ, Park EJ, Baik SH, Lee KY, Kang J.
ACKNOWLEDGMENTS
We would like to thank Editage (http://www.editage.co.kr) for English language editing.
References
1. Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010; 11:19–30.

3. Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019; 8:175–81.

4. Toiyama Y, Hiro J, Shimura T, Fujikawa H, Ohi M, Tanaka K, et al. The impact of body mass index on oncological outcomes in colorectal cancer patients with curative intent. Int J Clin Oncol. 2016; 21:1102–10.

5. Ding PQ, Batra A, Xu Y, McKinnon GP, Cheung WY. Obesity and its impact on outcomes in patients with stage III colon cancer receiving adjuvant chemotherapy. Clin Colorectal Cancer. 2020; 19:209–18.

6. Kalb M, Langheinrich MC, Merkel S, Krautz C, Brunner M, Benard A, et al. Influence of body mass index on long-term outcome in patients with rectal cancer: a single centre experience. Cancers (Basel). 2019; 11:609.

7. Arkenbosch JH, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JH, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol. 2019; 45:160–6.

8. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018; 8:620.

9. Daniel CR, Shu X, Ye Y, Gu J, Raju GS, Kopetz S, et al. Severe obesity prior to diagnosis limits survival in colorectal cancer patients evaluated at a large cancer centre. Br J Cancer. 2016; 114:103–9.

10. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016; 2:1137–45.

11. Fedirko V, Romieu I, Aleksandrova K, Pischon T, Trichopoulos D, Peeters PH, et al. Pre-diagnostic anthropometry and survival after colorectal cancer diagnosis in Western European populations. Int J Cancer. 2014; 135:1949–60.

12. Schlesinger S, Siegert S, Koch M, Walter J, Heits N, Hinz S, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014; 25:1407–18.

13. Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012; 30:42–52.

14. Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012; 21:1244–59.

15. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–63.
16. Yaghootkar H, Whitcher B, Bell JD, Thomas EL. Ethnic differences in adiposity and diabetes risk: insights from genetic studies. J Intern Med. 2020; 288:271–83.

17. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–40.
18. Song N, Huang D, Jang D, Kim MJ, Jeong SY, Shin A, et al. Optimal body mass index cut-off point for predicting colorectal cancer survival in an Asian population: a National Health Information Database analysis. Cancers (Basel). 2020; 12:830.

19. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio- informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10:7252–9.
20. Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015; 34:685–703.

21. Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016; 20:517–35.

22. Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016; 34:144–50.

23. Rim CH, Kim CY, Yang DS, Yoon WS. Clinical significance of gender and body mass index in Asian patients with colorectal cancer. J Cancer. 2019; 10:682–8.

24. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017; 26:1008–15.

25. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016; 57:58–67.

26. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016; 18:56.

27. Li L, Kalantar-Zadeh K. Obesity that makes kidney cancer more likely but helps fight it more strongly. J Natl Cancer Inst. 2013; 105:1848–9.

28. Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014; 62:96–102.

29. Ujvari B, Jacqueline C, Misse D, Amar V, Fitzpatrick JC, Jennings G, et al. Obesity paradox in cancer: Is bigger really better? Evol Appl. 2019; 12:1092–5.

30. Scarpa M, Ruffolo C, Erroi F, Fiorot A, Basato S, Pozza A, et al. Obesity is a risk factor for multifocal disease and recurrence after colorectal cancer surgery: a case-control study. Anticancer Res. 2014; 34:5735–41.
Fig. 1
Kaplan-Meier survival curve according to body mass index (BMI) category. Significant survival difference was observed among different BMI categories in patients with stage I–III colorectal cancer, in terms of overall survival (p < 0.001) (A) and recurrence-free survival (p=0.001) (B).
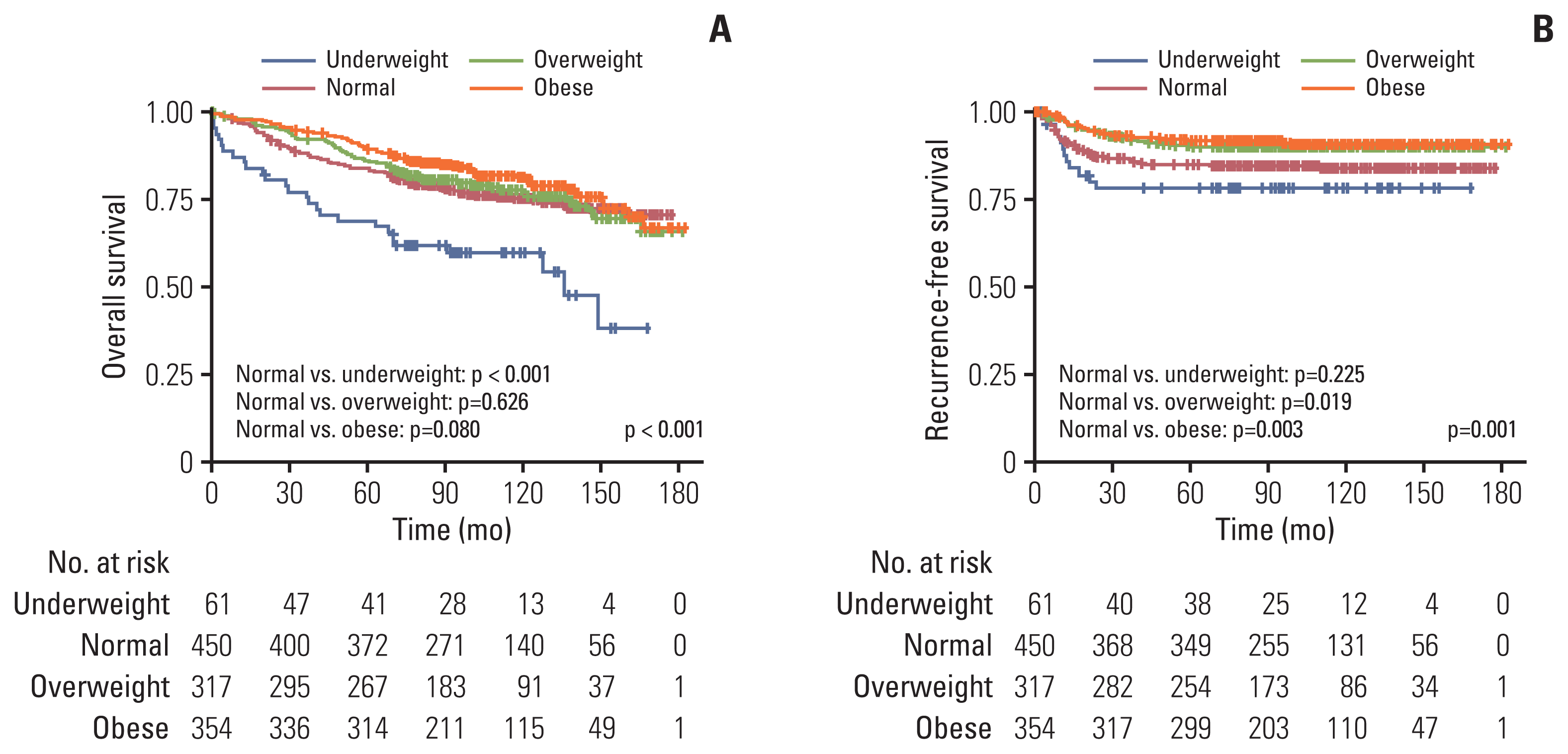
Fig. 2
Hazard ratio plot of preoperative body mass index (BMI) values. Risk of survival as a function of BMI are illustrated according to each sex subgroup for overall survival (OS) of all patients (A), men only (B), and women only (C), and recurrence-free survival (RFS) of all patients (D), men only (E), and women only (F). BMI was prognostic of OS and RFS in a reverse J-shaped curve, and this relationship varied by patient sex. The shaded area indicates 95% confidence intervals for the risk function.
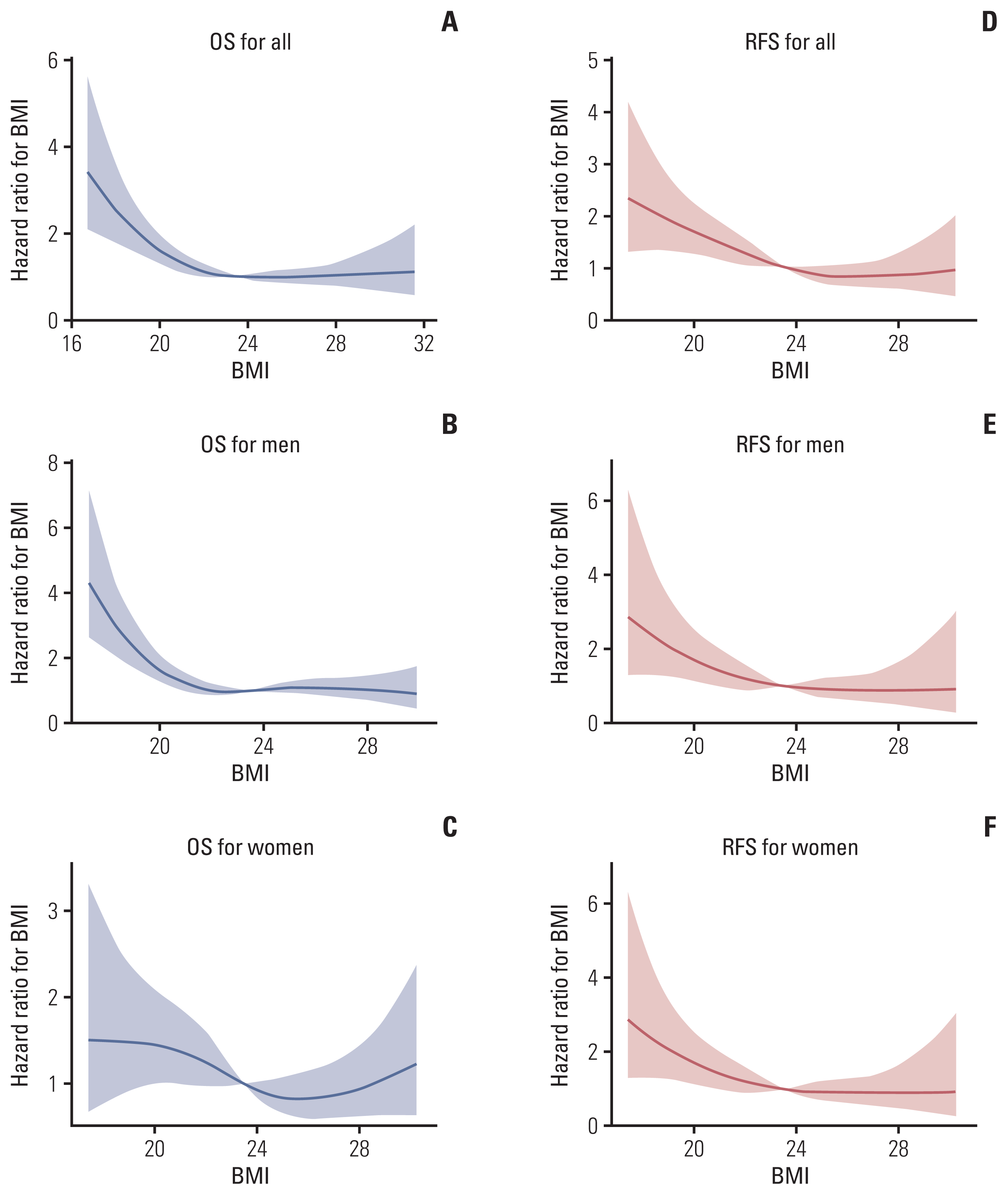
Fig. 3
Kaplan-Meier survival curve according to body mass index (BMI) cutoff value. Kaplan-Meier curves according to BMI cutoff value (20.44) in patients with stage I–III colorectal cancer for overall survival (p < 0.001) (A) and recurrence-free survival (p=0.004) (B).

Fig. 4
Integrated area under the curve (iAUC) between stage and stage plus body mass index (BMI) cutoff value for overall survival (A) and recurrence-free survival (B). The discriminatory power of continuous markers of overall survival and recurrence-free survival was assessed using the iAUC. The time-dependent receiver operating characteristic curve for stage+BMI cutoff value was superior to that of stage only for both overall survival (bootstrap iAUC mean difference, 0.047; 95% confidence interval [CI], 0.015 to 0.089) and recurrence-free survival (bootstrap iAUC mean difference, 0.043; 95% CI, 0.004 to 0.106) throughout follow-up.
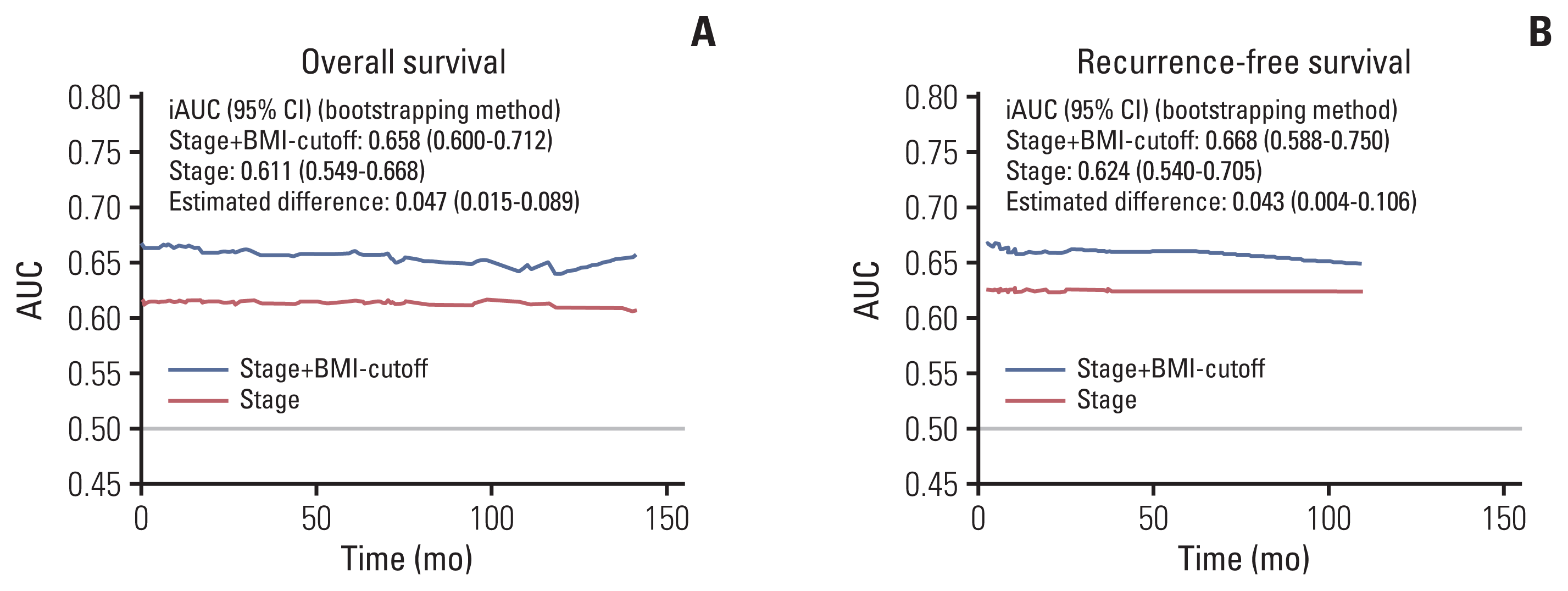
Table 1
Patient characteristics according to BMI groups
| Underweight (< 18.5 kg/m2) (n=61) | Normal (18.5–22.9 kg/m2) (n=450) | Overweight (23–24.9 kg/m2) (n=317) | Obese (≥ 25 kg/m2) (n=354) | p-valuea) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 29 (47.5) | 186 (41.3) | 123 (38.8) | 138 (39.0) | 0.556 |
| Male | 32 (52.5) | 264 (58.7) | 194 (61.2) | 216 (61.0) | |
| Age (yr) | |||||
| < 60 | 27 (44.3) | 166 (36.9) | 114 (36.0) | 131 (37.0) | 0.676 |
| ≥ 60 | 34 (55.7) | 284 (63.1) | 203 (64.0) | 223 (63.0) | |
| BMI (kg/m2) | 17.5±0.9 | 21.3±1.2 | 24.0±0.6 | 27.1±1.8 | < 0.001b) |
| DM | |||||
| No | 50 (82.0) | 362 (80.4) | 266 (83.9) | 277 (78.2) | 0.314 |
| Yes | 11 (18.0) | 88 (19.6) | 51 (16.1) | 77 (21.8) | |
| HTN | |||||
| No | 45 (73.8) | 299 (66.4) | 182 (57.4) | 167 (47.2) | < 0.001 |
| Yes | 16 (26.2) | 151 (33.6) | 135 (42.6) | 187 (52.8) | |
| CEA (ng/mL) | |||||
| < 5 | 38 (62.3) | 307 (68.2) | 220 (69.4) | 248 (70.1) | 0.919 |
| ≥ 5 | 20 (32.8) | 121 (26.9) | 80 (25.2) | 90 (25.4) | |
| Unknown | 3 (4.9) | 22 (4.9) | 17 (5.4) | 16 (4.5) | |
| Tumor location | |||||
| Rt. colon | 17 (27.9) | 123 (27.3) | 88 (27.8) | 76 (21.5) | 0.275 |
| Lt. colon | 29 (47.5) | 180 (40.0) | 138 (43.5) | 162 (45.8) | |
| Rectum | 15 (24.6) | 147 (32.7) | 91 (28.7) | 116 (32.8) | |
| Tumor size | |||||
| < 5 | 27 (44.3) | 269 (59.8) | 194 (61.2) | 236 (66.7) | 0.007 |
| ≥ 5 | 34 (55.7) | 181 (40.2) | 123 (38.8) | 118 (33.3) | |
| Complications | |||||
| No | 42 (68.9) | 335 (74.4) | 252 (79.5) | 270 (76.3) | 0.217 |
| Yes | 19 (31.1) | 115 (25.6) | 65 (20.5) | 84 (23.7) | |
| Histologic grade | |||||
| G1 & G2 | 50 (82.0) | 415 (92.2) | 295 (93.1) | 330 (93.2) | 0.021 |
| G3 & MC & SRC | 11 (18.0) | 35 (7.8) | 22 (6.9) | 24 (6.8) | |
| LVI | |||||
| Absent | 40 (65.6) | 282 (62.7) | 221 (69.7) | 247 (69.8) | 0.043 |
| Present | 14 (23.0) | 108 (24.0) | 53 (16.7) | 53 (15.0) | |
| Unknown | 7 (11.5) | 60 (13.3) | 43 (13.6) | 54 (15.3) | |
| Retrieved LNs | |||||
| < 12 | 6 (9.8) | 63 (14.0) | 54 (17.0) | 76 (21.5) | 0.017 |
| ≥ 12 | 55 (90.2) | 387 (86.0) | 263 (83.0) | 278 (78.5) | |
| Stage | |||||
| I | 12 (19.7) | 101 (22.4) | 84 (26.5) | 112 (31.6) | 0.055 |
| II | 18 (29.5) | 158 (35.1) | 108 (34.1) | 116 (32.8) | |
| III | 31 (50.8) | 191 (42.4) | 125 (39.4) | 126 (35.6) | |
| Chemotherapy | |||||
| No | 29 (47.5) | 168 (37.3) | 118 (37.2) | 147 (41.5) | 0.289 |
| Yes | 32 (52.5) | 282 (62.7) | 199 (62.8) | 207 (58.5) | |
Table 2
Univariable and multivariable analysis of factors associated with overall survival (n=1,182)
Table 3
Univariable and multivariable analysis of factors associated with recurrence-free survival (n=1,182)
Table 4
Comparison of C-index between stage versus stage and BMI-cutoff in the test set (n=355)
Table 5
Summary of hazard ratio and 95% confidence interval in patients with colorectal cancer
| Study/Nation | No./Outcome | Stage | BMI (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| < 18.5 | 18.5–22.9 | 23–24.9 | 25–27.49 | 27.5–29.9 | 30–34.9 | 35–39.9 | ≥ 40 | |||
| This study/Korea Ding et al. (2020) | 1,182/OS | Stage 1–3 | BMI < 18.5: 2.38 (1.55–3.66) | Ref | 0.91 (0.63–1.23) | 25 ≤ BMI < 35: 0.72 (0.53–0.97) | BMI ≥ 35: N/A | |||
|
|
||||||||||
| Ding et al. (2020) [5]/Canada | 915/OS | Stage 3 | BMI < 18.5: 1.29 (0.77–2.19) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.88 (0.55–1.41) | BMI ≥ 30: 1.18 (0.63–2.20) | ||||
|
|
||||||||||
| Kalb et al. (2019) [6]/Germany | 598/OS | (y) Stage 1–3 (Rectal cancer only) | BMI < 18.5: 1.7 (0.4–7.2) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 1.2 (0.8–1.7) | 0.7 (0.5–1.2) | 1.4 (0.7–2.9) | 0.7 (0.2–2.0) | ||
|
|
||||||||||
| Arkenbosch et al. (2019) [7]/Netherlands | 7,371/OS | Stage 1–3 | BMI < 18.5: 2.06 (1.51–2.80) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.86 (0.76–0.97) | BMI ≥ 30: 1.01 (0.87–1.17) | ||||
| Stage 4 | BMI < 18.5: 1.65 (1.11–2.45) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.85 (0.75–0.95) | BMI ≥ 30: 0.76 (0.64–0.91) | ||||||
|
|
||||||||||
| Shahjehan et al. (2018) [8]/USA | 3,799/OS | Stage 1 | BMI < 18.5: 2.4 (1.0–6.0) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.9 (0.7–1.2) | BMI ≥ 30: 1.2 (1.0–1.6) | ||||
| Stage 2 | BMI < 18.5: 1.8 (0.9–3.5) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.8 (0.6–1.0) | BMI ≥ 30: 0.9 (0.7–1.2) | ||||||
| Stage 3–4 | BMI < 18.5: 2.0 (1.2–3.2) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 1.0 (0.81–1.15) | BMI ≥ 30: 0.9 (0.8–1.1) | ||||||
|
|
||||||||||
| Daniel et al. (2016) [9]/USA | 409/OS | Stage 1–3 | BMI < 25: 1.59 (0.74–3.43) | 25 ≤ BMI < 30: | 30 ≤ BMI < 35: 2.21 (1.11–4.40) | BMI ≥ 35: 3.49 (1.68–7.22) | ||||
|
|
||||||||||
| Kroenke et al. (2016) [10]/USA | 3,408/OS | Stage 1–3 | BMI < 18.5: 3.01 (1.88–4.83) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.81 (0.64–1.03) | :30 ≤ BMI < 40: 1.03 (0.77–1.38) | BMI ≥ 40: 1.63 (1.13–2.33) | |||
|
|
||||||||||
| Fedirko et al. (2014) [11]/Europe | 3,924/OS | Stage 1–4 | BMI < 25: Ref | 25 ≤ BMI < 35: 1.12 (0.97–1.30) | BMI ≥ 30: 1.26 (1.04–1.52) | |||||
|
|
||||||||||
| Schlesinger et al. (2014) [12]/Germany | 2,143/OS | NA | BMI < 18.5: 1.65 (0.79–3.46) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.80 (0.62–1.02) | BMI ≥ 30: 0.84 (0.62–1.14) | ||||
|
|
||||||||||
| Campbell et al. (2012) [13]/USA | 2,303/CSS | NA | BMI < 18.5: 0.67 (0.21–2.12)a) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 1.09 (0.85–1.40)a) | BMI ≥ 30: 1.35 (1.01–1.80)a) | ||||
| BMI < 18.5: 0.64 (0.25–1.60)b) | 18.5 ≤ BMI < 25: Ref | 25 ≤ BMI < 35: 0.87 (0.65–1.17)b) | BMI ≥ 30: 1.14 (0.81–1.60)b) | |||||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download