Abstract
Purpose
Materials and Methods
Results
Conclusion
Notes
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Ethical Statement
After the approval of the Institutional Review Board off the three institutions (No. J-2004-131-1117; B-2005/612-403; 20200327/20-2020-28/042). Because of the retrospective design of the analysis, the requirement to obtain informed consent of participants included in the study was exempted.
Author Contributions
Conceived and designed the analysis: Kim DY, Wu HG, Kim JH, Lee JH, Wee CW.
Collected the data: Kim DY, Wu HG, Kim JH, Lee JH, Ahn SH, Chung EJ, Eom KY, Jung YH, Jeong WJ, Kwon TK, Kim S, Wee CW.
Contributed data or analysis tools: Kim DY, Wu HG, Kim JH, Lee JH, Ahn SH, Chung EJ, Eom KY, Jung YH, Jeong WJ, Kwon TK, Kim S, Wee CW.
Performed the analysis: Kim DY, Wee CW.
Wrote the paper: Kim DY, Wee CW.
References
Fig. 1
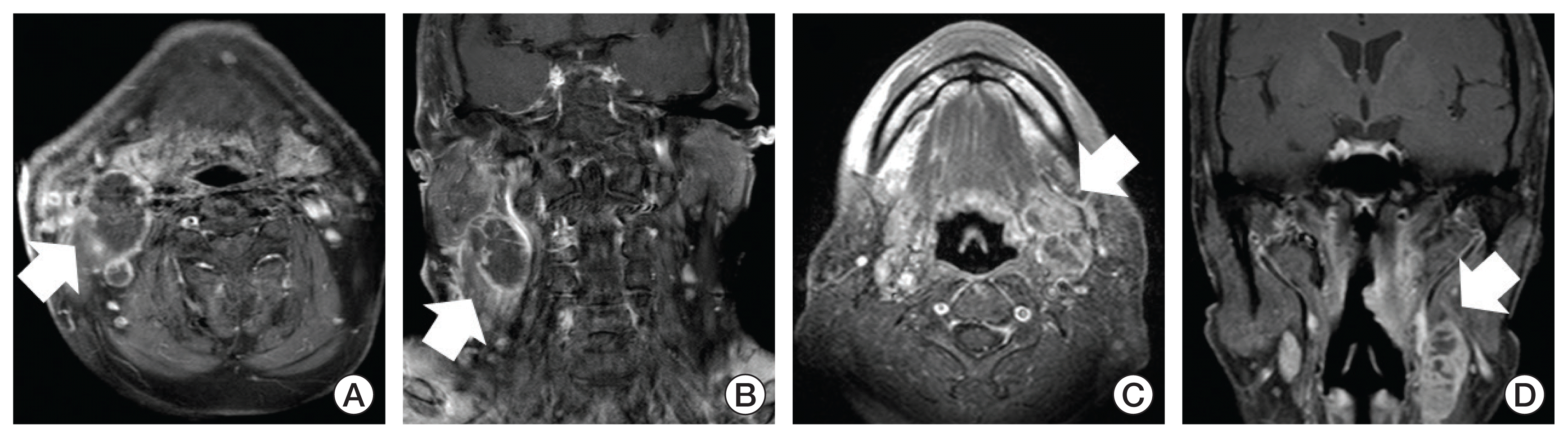
Fig. 2
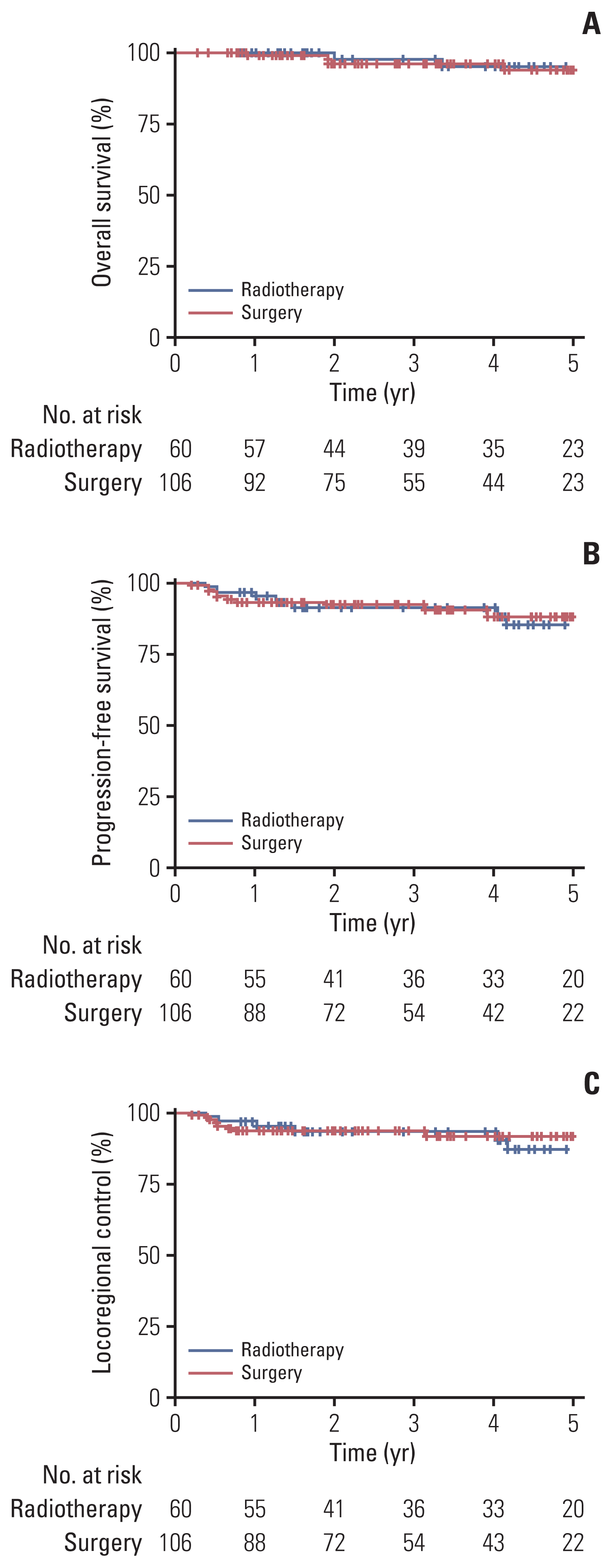
Table 1
| Characteristic | Total (n=166) | RT (n=60) | Surgery (n=106) | p-valuea) |
|---|---|---|---|---|
| Age (yr) | 58.5 (36–86) | 59 (46–77) | 58 (36–86) | 0.559b) |
| Age (yr) | ||||
| < 60 | 93 (56.0) | 32 (53.3) | 61 (57.5) | 0.599 |
| ≥ 60 | 73 (44.0) | 28 (46.7) | 45 (42.5) | |
| Sex | ||||
| Male | 139 (83.7) | 48 (80.0) | 91 (85.8) | 0.327 |
| Female | 27 (16.3) | 12 (20.0) | 15 (14.2) | |
| Primary site | ||||
| Tonsil | 143 (86.1) | 50 (83.3) | 93 (87.7) | 0.678 |
| Othersc) | 23 (13.9) | 10 (16.7) | 13 (12.3) | |
| ACCI | ||||
| 1–5 | 157 (94.6) | 56 (93.3) | 101 (95.3) | 0.594 |
| 6–9 | 9 (5.4) | 4 (6.7) | 5 (4.7) | |
| Smoking history | ||||
| ≤ 10 pack-years | 77 (46.4) | 36 (60.0) | 41 (38.7) | 0.008 |
| > 10 pack-years | 89 (53.6) | 24 (40.0) | 65 (61.3) | |
| Clinical T category | ||||
| T0–T1 | 44 (26.5) | 6 (10.0) | 38 (35.8) | < 0.001 |
| T2–T3 | 122 (73.5) | 54 (90.0) | 68 (64.2) | |
| Clinical N category | ||||
| N0 | 17 (10.2) | 3 (5.0) | 14 (13.2) | 0.003 |
| N1 | 132 (79.5) | 45 (75.0) | 87 (82.1) | |
| N2 | 17 (10.2) | 12 (20.0) | 5 (4.7) | |
| Clinical stage (AJCC 8th) | ||||
| I | 144 (86.7) | 47 (78.3) | 97 (91.5) | 0.016 |
| II | 22 (13.3) | 13 (21.7) | 9 (8.5) | |
| Metastatic LN with clinical extranodal extension | ||||
| No | 91 (54.8) | 32 (53.3) | 59 (55.7) | 0.265 |
| Yes | 75 (45.2) | 28 (46.7) | 47 (44.3) | |
Table 2
| Overall survival | Progression-free survival | Locoregional control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
|
|
|
|
|
|
|
|||||||
| 2-Year rate (%) | p-valuea) | HR (95% CI) | p-valueb) | 2-Year rate (%) | p-valuea) | HR (95% CI) | p-valueb) | 2-Year rate (%) | p-valuea) | HR (95% CI) | p-valueb) | |
| Overall | 96.9 | 91.6 | 93.1 | |||||||||
|
|
||||||||||||
| Age (yr) | ||||||||||||
|
|
||||||||||||
| < 60 | 94.9 | 0.237 | 1.00 | 0.204 | 88.5 | 0.230 | 1.00 | 0.087 | 91.0 | 0.238 | 1.00 | 0.083 |
|
|
||||||||||||
| ≥ 60 | 100 | 5.06 (0.57–45.17) | 95.9 | 0.37 (0.12–1.16) | 95.9 | 0.32 (0.09–1.16) | ||||||
|
|
||||||||||||
| Sex | ||||||||||||
|
|
||||||||||||
| Male | 96.3 | 0.287 | 1.00 | 0.986 | 90.0 | 0.064 | 1.00 | 0.974 | 91.8 | 0.093 | 1.00 | 0.976 |
|
|
||||||||||||
| Female | 100 | N/Ac) | 100 | N/Ac) | 100 | N/Ac) | ||||||
|
|
||||||||||||
| Primary site | ||||||||||||
|
|
||||||||||||
| Tonsil | 96.5 | 0.331 | 1.00 | 0.981 | 91.1 | 0.317 | 1.00 | 0.162 | 92.0 | 0.124 | 1.00 | 0.979 |
|
|
||||||||||||
| Othersd) | 100 | N/Ac) | 94.7 | 0.20 (0.02–1.89) | 100 | N/Ac) | ||||||
|
|
||||||||||||
| ACCI | ||||||||||||
|
|
||||||||||||
| 1–5 | 96.8 | 0.613 | 1.00 | 0.996 | 91.8 | 0.903 | 1.00 | 0.222 | 93.3 | 0.766 | 1.00 | 0.199 |
|
|
||||||||||||
| 6–9 | 100 | N/Ac) | 88.9 | 4.29 (0.42–44.25) | 88.9 | 4.84 (0.44–53.65) | ||||||
|
|
||||||||||||
| Smoking pack-year | ||||||||||||
|
|
||||||||||||
| < 10 | 95.4 | 0.404 | 1.00 | 0.638 | 93.0 | 0.503 | 1.00 | 0.572 | 95.9 | 0.331 | 1.00 | 0.314 |
|
|
||||||||||||
| ≥ 10 | 98.4 | 0.66 (0.11–3.78) | 90.7 | 1.34 (0.48–3.75) | 90.7 | 0.551 (0.17–1.76) | ||||||
|
|
||||||||||||
| Clinical stage (AJCC 8th) | ||||||||||||
|
|
||||||||||||
| I | 98.3 | 0.059 | 1.00 | 0.019 | 92.8 | 0.450 | 1.00 | 0.253 | 92.8 | 0.577 | 1.00 | 0.986 |
|
|
||||||||||||
| II | 84.6 | 10.94 (1.49–80.42) | 82.2 | 2.07 (0.50–8.54) | 95.2 | 0.98 (0.12–8.06) | ||||||
|
|
||||||||||||
| Metastatic LN with clinical extranodal extension | ||||||||||||
|
|
||||||||||||
| No | 100 | 0.140 | 1.00 | 0.146 | 97.1 | 0.002 | 1.00 | 0.006 | 97.1 | 0.010 | 1.00 | 0.015 |
|
|
||||||||||||
| Yes | 94.3 | 5.06 (0.57–45.17) | 86.9 | 8.23 (1.84–36.76) | 89.6 | 7.28 (2.01–26.40) | ||||||
|
|
||||||||||||
| Primary treatment | ||||||||||||
|
|
||||||||||||
| Radiotherapy | 97.8 | 0.755 | 1.00 | 0.683 | 91.1 | 0.810 | 1.00 | 0.530 | 92.9 | 0.721 | 1.00 | 0.294 |
|
|
||||||||||||
| Surgery | 96.4 | 1.49 (0.22–9.93) | 92.0 | 0.71 (0.25–2.05) | 93.3 | 0.541 (0.17–1.71) | ||||||
Table 3
| Variable | Overall survival | Progression-free survival | Locoregional control | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI) | p-valuea) | HR (95% CI) | p-valuea) | HR (95% CI) | p-valuea) | |
| Age (yr) | ||||||
|
|
||||||
| < 60 | 1.00 | 0.375 | 1.00 | 0.156 | 1.00 | 0.144 |
|
|
||||||
| ≥ 60 | 2.70 (0.30–24.16) | 2.30 (0.73–7.29) | 2.64 (0.72–9.75) | |||
|
|
||||||
| Sex | ||||||
|
|
||||||
| Male | 1.00 | 0.981 | 1.00 | 0.975 | 1.00 | 0.977 |
|
|
||||||
| Female | N/Ab) (N/A) | N/Ab) (N/A) | N/Ab) (N/A) | |||
|
|
||||||
| Primary site | ||||||
|
|
||||||
| Tonsil | 1.00 | 0.972 | 1.00 | 0.163 | 1.00 | 0.980 |
|
|
||||||
| Othersc) | N/Ab) (N/A) | 0.21 (0.02–1.88) | N/Ab) (N/A) | |||
|
|
||||||
| ACCI | ||||||
|
|
||||||
| 1–5 | 1.00 | 0.993 | 1.00 | 0.353 | 1.00 | 0.315 |
|
|
||||||
| 6–9 | N/Ab) (N/A) | 2.97 (0.30–29.57) | 3.34 (0.32–35.10) | |||
|
|
||||||
| Smoking history | ||||||
|
|
||||||
| ≤ 10 pack-years | 1.00 | 0.639 | 1.00 | 0.618 | 1.00 | 0.393 |
|
|
||||||
| > 10 pack-years | 1.52 (0.27–8.65) | 1.30 (0.46–3.64) | 1.67 (0.52–5.37) | |||
|
|
||||||
| Clinical stage (AJCC 8th) | ||||||
|
|
||||||
| I | 1.00 | 0.018 | 1.00 | 0.316 | 1.00 | 0.875 |
|
|
||||||
| II | 13.21 (1.55–112.63) | 2.07 (0.50–8.54) | 0.84 (0.10–7.01) | |||
|
|
||||||
| Metastatic LN with clinical extranodal extension and conglomeration | ||||||
|
|
||||||
| No | 1.00 | 0.047 | 1.00 | 0.001 | 1.00 | 0.003 |
|
|
||||||
| Yes | 9.77 (1.03–92.50) | 9.18 (2.62–32.12) | 7.28 (2.01–26.40) | |||
|
|
||||||
| Primary treatment | ||||||
|
|
||||||
| Radiotherapy | 1.00 | 0.460 | 1.00 | 0.709 | 1.00 | 0.433 |
|
|
||||||
| Surgery | 2.12 (0.29–15.44) | 0.82 (0.28–2.36) | 0.63 (0.19–2.02) | |||
Table 4
Table 5
| Postoperative RT | Postoperative CCRT | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p-valuea) | OR | 95% CI | p-valuea) | |
| Clinical T category | ||||||
|
|
||||||
| T0/1 | 1.00 | 0.765 | 1.00 | 0.317 | ||
|
|
||||||
| T2/3 | 1.16 | 0.43–3.11 | 1.66 | 0.61–4.50 | ||
|
|
||||||
| Clinical N category | ||||||
|
|
||||||
| N0/1 | 1.00 | NA | 1.00 | 0.913 | ||
|
|
||||||
| N2 | NA | NA | 1.13 | 0.13–9.81 | ||
|
|
||||||
| No. of clinically positive LNs | ||||||
|
|
||||||
| < 2 | 1.00 | 0.506 | 1.00 | 0.004 | ||
|
|
||||||
| ≥ 2 | 1.55 | 0.42–5.69 | 5.15 | 1.68–15.74 | ||
|
|
||||||
| Maximum size of clinically positive LN (mm) | ||||||
|
|
||||||
| < 20 | 1.00 | 0.017 | 1.00 | 0.138 | ||
|
|
||||||
| ≥ 20 | 4.34 | 1.31–14.45 | 2.61 | 0.73–9.29 | ||
|
|
||||||
| Metastatic LN with clinical extranodal extension | ||||||
|
|
||||||
| No | 1.00 | 0.650 | 1.00 | 0.650 | ||
|
|
||||||
| Yes | 0.72 | 0.18–2.96 | 0.66 | 0.18–2.96 | ||
|
|
||||||
| Extranodal extension with conglomeration | ||||||
|
|
||||||
| No | 1.00 | 0.330 | 1.00 | 0.019 | ||
|
|
||||||
| Yes | 1.95 | 0.51–7.52 | 4.83 | 1.30–17.95 | ||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download