Introduction
Materials and Methods
1. Study design
2. Cell production
3. Patient evaluation
4. Cytokine analysis
5. Statistical analysis
Results
Table 1
| Patient ID | Sex | Age (yr) | Tissue involvement | Enrollment criteria | Time from initial diagnosis to CAR-T infusion (mo) | Genetic variation | Minimal residual disease before CAR-T (%) | Infusion dose of 19CAR-T (×106/kg) | CRS grade | CRES grade | IL-6 max (pg/mL) | CNS status at the time of CAR-T cell infusion | Disease status on day 28a) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S001 | M | 4.3 | BM, subcutaneous tumor | Relapse | 7.50 | TCF3-PBX1 | 95.83 | 6.9 | 1b) | 0 | 390.25 | ND | MRD Neg, 30×21 mmc) |
| S002 | F | 7.2 | BM and CNS | 2nd Relapse | 44.07 | - | 0.27 | 10 | 1 | 1 | 100.39 | ND | MRD Neg |
| S003 | F | 6.1 | BM and CNS after HSCT | 2nd Relapse | 50.10 | ETV6-RUNX1 | 31.35 | 12 | 3b),d) | 0 | 60,618.45 | ND | MRD Neg |
| S004 | F | 8.7 | BM and CNS | 2nd Relapse | 72.87 | ZCCHC7-CSF2RA | 2.41 | 15 | 1d) | 1 | 1,272.27e) | Mild Headache | MRD Neg |
| S005 | M | 9.3 | BM and testis | Relapse | 40.10 | ETV6-RUNX1 | 1.00 | 15 | 3b),d) | 2 | 1,445.5 | Drowsiness and slow response to stimuli | - |
| S006 | F | 7.4 | BM, subcutaneous tumor | Relapse | 44.17 | ND | 0.46 | 10 | 0 | 0 | 96.4 | ND | - |
| S007 | M | 7.1 | BM, CNS, and testis | 2nd Relapse | 48.27 | - | 3.00 | 8.8 | 2b) | 2 | 13,116.22 | Limb trembling and delirium | MRD Neg |
| S008 | F | 13 | BM and CNS | 2nd Relapse | 116.83 | ETV6: exon 2 del | 32.40 | 1.8 | 2b) | 2 | 574.7e) | Unconsciousness | MRD Neg |
S004 received glucocorticoids by intrathecal injection. -, no follow-up; 19CAR-T, CD19 chimeric antigen receptor T-cell; BM, bone marrow; CAR-T, chimeric antigen receptor T-cell; CNS, central nervous system; CRES, CAR-T related encephalopathy syndrome; CRS, cytokine release syndrome; F, female; HSCT, hematopoietic stem cell transplantation; IL-6, interleukin 6; M, male; MRD, minimal residual disease; ND, not detected; Neg, negative.
c) According to the results of ultrasound, patient S001 was diagnosed with a 45×25 mm enlarged lymph node near an iliac vessel in the pelvis before CAR-T infusion; the dimensions decreased to 30×21 mm after 28 days, and after 3 months, only one 18×10 mm lymph node was found in the right inguinal region,
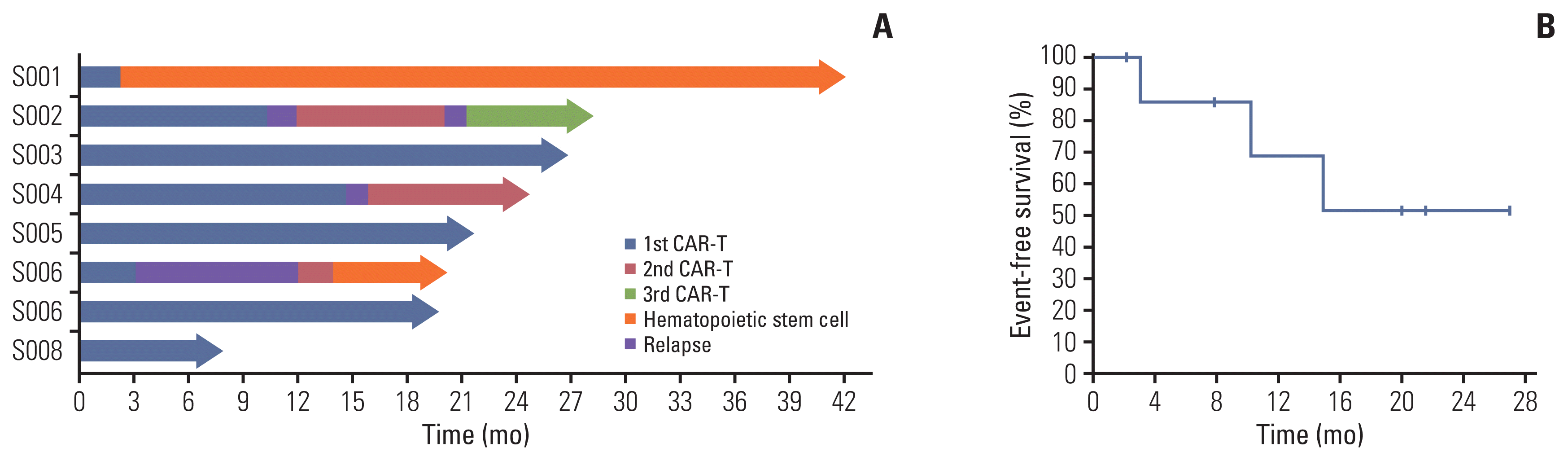 | Fig. 1Clinical outcomes of 19CAR-T cells. (A) Swimmer plot (n=8), in which each bar represents an individual patient as designated. (B) Kaplan-Meier graph of event-free survival in eight patients infused with 19CAR-T cells, demonstrating 68.57% event-free survival at 12 months and 51.43% at 24 months. 19CAR-T, anti-CD19 chimeric antigen receptor T-cell; CAR-T, chimeric antigen receptor T-cell. |
 | Fig. 2Changes in patients’ serum biomarkers in cytokine release syndrome. (A) The temporal relationship of interleukin 6 (IL-6) (blue line) and interferon γ (IFN-γ) (red line) from day 0 to day 11. (B) The temporal relationship of IL-6 (blue line) and lymphocyte numbers (red line) from day 0 to day 8. (C) The number of copies of the chimeric antigen receptor (CAR) gene detected in Patient S005’s peripheral blood (PB) and cerebrospinal fluid(CSF). (D) The persistence of circulating chimeric antigen receptor T-cell (CAR-T) cells identified by quantitative polymerase chain reaction (qPCR). (E) B-cell aplasia remained in patient S003 but not in S001, who had been bridged to hematopoietic stem cell transplantation. (F) There were 90.7% leukemic cells in bone marrow from patient S003 detected on day 0 (left) but 0.0% on day 21 (right). |
Table 2
| Patient ID | 2nd relapse sites | CD19/CD22 expression after 2nd relapse (%) | Infusion dose of 19/22CAR-T (×106 /kg) | 19CAR-T in all (%) | CRS grade | CRES grade | IL-6 max (pg/mL) | Responses | B cell on day 7 after CAR-T infusion (cells/μL) | Bridge to HSCT |
|---|---|---|---|---|---|---|---|---|---|---|
| S002 | BM | - | 15 | 60.0 | 1 | 0 | 24.17 | CRa) | 0.0 | N |
| S004 | BM | 98.3/99.7 | 4.26 | 51.3 | 1 | 0 | - | CR | - | N |
| S006 | Subcutaneous tumor | 100/100 | 12 | 20.6 | 1 | 0 | - | CR | - | Y |
-, no follow-up; 19CAR-T, anti-CD19 chimeric antigen receptor T-cell; BM, bone marrow; CAR-T, chimeric antigen receptor T-cell; CR, complete remission; CRES, CAR-T related encephalopathy syndrome; CRS, cytokine release syndrome; HSCT, hematopoietic stem cell transplantation; IL-6, interleukin 6; N, no; Y, yes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download