Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
The local Ethics and Research Committees approved the study (No. 2019/0021). This is a retrospective study, approved by the Ethics and Research Comittees. For this retrospective study, the informed consent from the patient was not required. All the information used in this study was collected from the medical records. However, all patients that undergo cancer treatment at our Institution sign an infomed consent, in which they express their agreement to receive the treatment proposed by their physician.
Author Contributions
Conceived and designed the analysis: Cetina-Pérez L.
Collected the data: Brau-Figueroa H, Arango-Bravo E, Galicia-Carmona T, Abraham Lugo-Alferez L.
Contributed data or analysis tools: Brau-Figueroa H, Jiménez-Lima R.
Performed the analysis: Brau-Figueroa H, Arango-Bravo E, Castro-Eguiluz D.
Wrote the paper: Brau-Figueroa H, Arango-Bravo E, Castro-Eguiluz D.
Manuscript editing: Castro-Eguiluz D.
Guarantor of integrity of study: Cruz-Bautista I.
References
Fig. 1
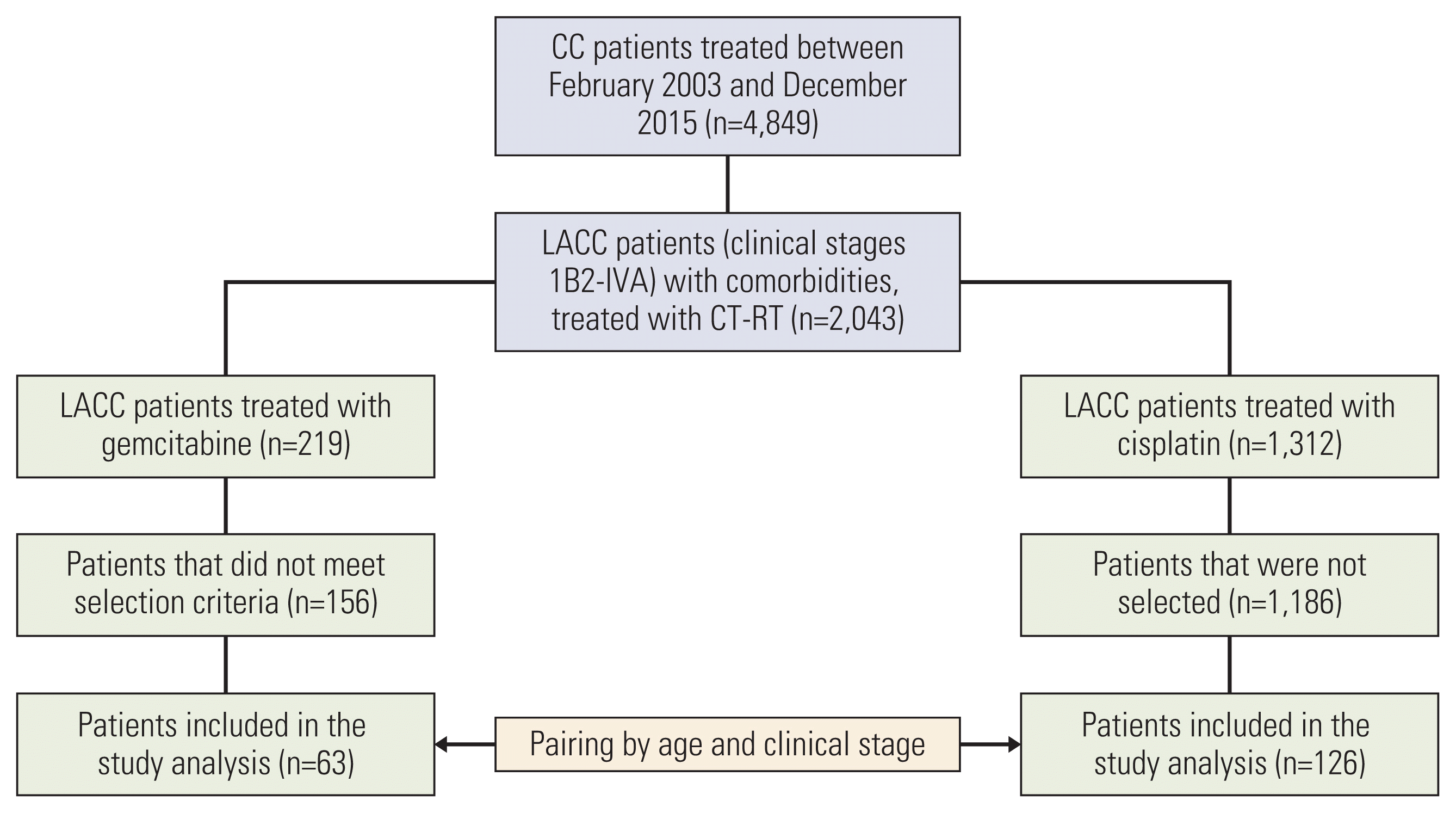
Fig. 2
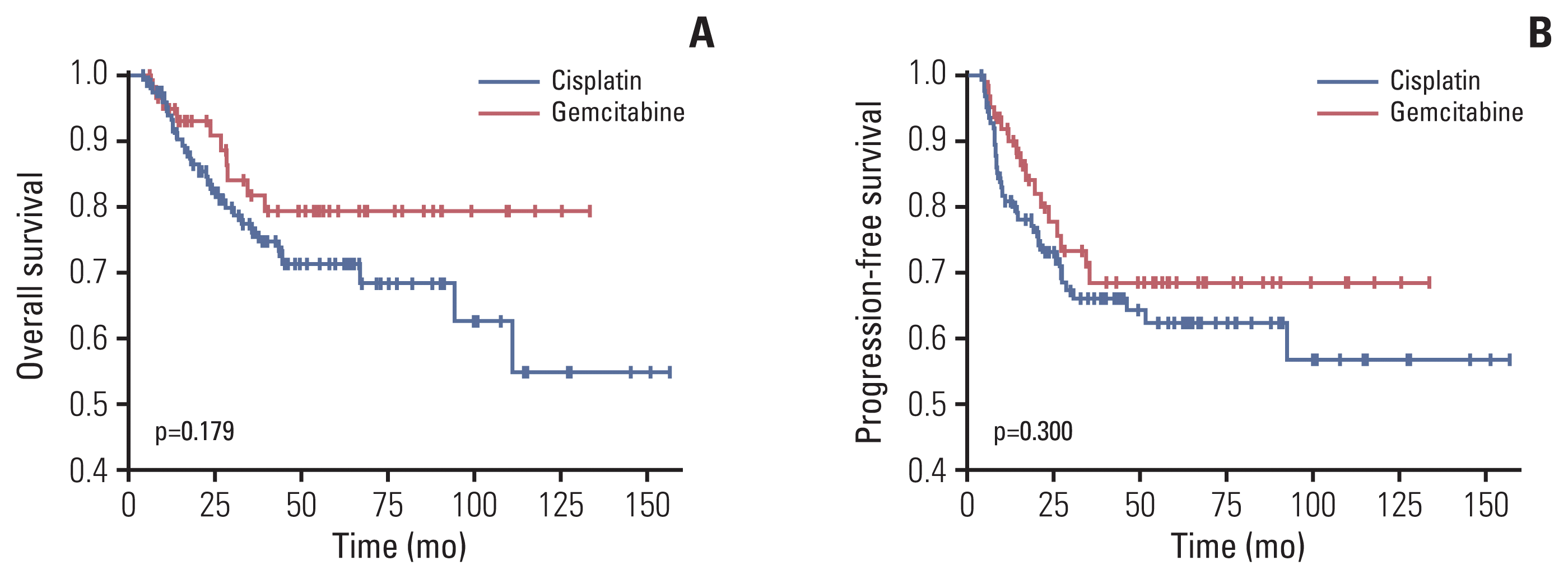
Table 1
| Characteristic | Total patients (n=189) | Cisplatin (n=126) | Gemcitabine (n=63) | p-value |
|---|---|---|---|---|
| Age (yr)a) | 56.3±11.5 | 55.4±10.9 | 57.9±12.5 | 0.668b) |
| Performance statusc) | ||||
| ECOG 0 | 77 (40.7) | 47 (37.3) | 30 (47.6) | 0.348d) |
| ECOG 1 | 107 (56.6) | 76 (60.3) | 31 (49.2) | |
| ECOG 2 | 5 (2.6) | 3 (2.4) | 2 (3.2) | |
| Clinical stagee) | ||||
| IB2 | 12 (6.3) | 8 (6.3) | 4 (6.3) | 0.975d) |
| IIA | 6 (3.2) | 4 (3.2) | 2 (3.2) | |
| IIB | 108 (57.1) | 72 (57.1) | 36 (57.1) | |
| IIIA | 1 (0.5) | 1 (0.8) | 0 | |
| IIIB | 59 (31.2) | 39 (31.0) | 20 (31.7) | |
| IVB | 3 (1.6) | 2 (1.6) | 1 (1.6) | |
| Hemoglobin (g/dL) | 12.8±2.04 | 12.9±2.02 | 12.7±2.05 | 0.574b) |
| Histology | ||||
| Squamous cell carcinoma | 157 (83.1) | 102 (81.0) | 55 (87.3) | 0.536d) |
| Adenocarcinoma | 23 (12.2) | 17 (13.5) | 6 (9.5) | |
| Adenosquamous carcinoma | 9 (4.8) | 7 (5.6) | 2 (3.2) | |
| Comorbidities | ||||
| Number | 1.24±0.48 | 1.23±0.50 | 1.26±0.44 | 0.051b) |
| Comorbidities=1 | 148 (78.3) | 102 (80.9) | 46 (73.9) | 0.212d) |
| Comorbidities ≥ 2 | 41 (21.6) | 24 (19.0) | 17 (26.9) | |
| T2DM | 89 (46.8) | 57 (45.2) | 32 (50.8) | < 0.001d) |
| SAH | 112 (58.9) | 81 (64.3) | 31 (49.2) | |
| Acute kidney injury | 19 (10.0) | 14 (11.1) | 5 (7.9) | |
| Other | 15 (7.9) | 3 (2.4) | 12 (19.0) | |
| Response ratesf) | ||||
| Complete response | 165 (87.3) | 109 (86.5) | 56 (88.9) | 0.094d) |
| Partial response | 9 (4.7) | 4 (3.2) | 5 (7.9) | |
| Disease progression | 15 (7.9) | 13 (10.3) | 2 (3.2) | |
| Pattern of failure | ||||
| Local | 12 (6.3) | 7 (5.5) | 5 (7.9) | 0.917d) |
| Locoregional | 9 (4.7) | 6 (4.7) | 3 (4.7) | |
| Distant metastasis | 35 (18.5) | 22 (17.4) | 13 (20.6) | |
| Locoregional+distant metastasis | 16 (8.4) | 14 (11.1) | 2 (3.1) | |
| Persistent disease | 13 (6.8) | 10 (7.9) | 3 (4.7) | |
Table 2
| Toxicity | Cisplatin (n=126) | Gemcitabine (n=63) | p-valueb) | ||
|---|---|---|---|---|---|
|
|
|
||||
| G1–2 | G3–4 | G1–2 | G3–4 | ||
| Neutropenia | 57 (45.2) | 7 (5.5) | 24 (38.0) | 4 (6.3) | 0.646 |
|
|
|||||
| Leukopenia | 98 (77.7) | 13 (10.3) | 29 (46.0) | 3 (4.8) | < 0.001 |
|
|
|||||
| Lymphopenia | 70 (55.5) | 35 (27.8) | 9 (14.3) | 1 (1.6) | < 0.001 |
|
|
|||||
| Anemia | 22 (17.4) | - | 11 (17.4) | 2 (3.2) | 0.132 |
|
|
|||||
| Thrombocytopenia | 7 (5.5) | - | 4 (6.3) | - | 0.530 |
|
|
|||||
| Nausea | 110 (87.3) | 7 (5.6) | 59 (93.6) | - | 0.155 |
|
|
|||||
| Vomit | 69 (54.7) | 4 (3.2) | 49 (77.7) | - | 0.003 |
|
|
|||||
| Constipation | 14 (11.1) | - | 18 (28.5) | - | 0.003 |
|
|
|||||
| Diarrhea | 63 (50.0) | 5 (4.0) | 44 (69.8) | 8 (12.7) | < 0.001 |
|
|
|||||
| Pruritus | 3 (2.4) | - | 3 (4.7) | - | 0.318 |
|
|
|||||
| Fever | 3 (2.4) | - | 11 (17.5) | - | < 0.001 |
|
|
|||||
| Neuropathy | 8 (6.3) | - | 1 (1.6) | - | 0.047 |
|
|
|||||
| Cephalea | 9 (7.1) | - | 5 (7.9) | - | 0.522 |
|
|
|||||
| Anorexia | 3 (2.4) | - | 3 (4.7) | 1 (1.6) | 0.244 |
|
|
|||||
| Fatigue | 71 (56.3) | - | 55 (87.3) | 1 (1.6) | < 0.001 |
|
|
|||||
| Edema | 2 (1.6) | - | 5 (7.9) | - | 0.042 |
|
|
|||||
| Abdominal pain | 37 (29.3) | - | 27 (42.8) | - | 0.065 |
|
|
|||||
| Non-infectious cystitis | 23 (18.2) | 1 (0.8) | 27 (42.8) | - | 0.001 |
|
|
|||||
| Proctitis | 14 (11.1) | 10 (7.9) | 4 (6.3) | 3 (4.8) | 0.381 |
|
|
|||||
| Dermal toxicity | 14 (11.1) | 1 (0.8) | 3 (4.7) | 1 (1.6) | 0.516 |
Table 3
| Treatment group | Pre-treatment | Post-treatment (1 year) | ΔGFR | p-valueb) |
|---|---|---|---|---|
| Cisplatin | 94.5 | 90.9 | −3.6 | 0.002 |
| Gemcitabine | 93.2 | 95.1 | +1.9 | 0.667 |
| p=0.025c) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download