1. DeNunzio NJ, Yock TI. Modern radiotherapy for pediatric brain tumors. Cancers (Basel). 2020; 12:1533.

2. Song S, Park HJ, Yoon JH, Kim DW, Park J, Shin D, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014; 53:1158–64.

3. Seravalli E, Bosman M, Lassen-Ramshad Y, Vestergaard A, Oldenburger F, Visser J, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy working group). Acta Oncol. 2018; 57:1240–9.

4. Cox MC, Kusters JM, Gidding CE, Schieving JH, van Lindert EJ, Kaanders JH, et al. Acute toxicity profile of craniospinal irradiation with intensity-modulated radiation therapy in children with medulloblastoma: a prospective analysis. Radiat Oncol. 2015; 10:241.

5. Chang EL, Allen P, Wu C, Ater J, Kuttesch J, Maor MH. Acute toxicity and treatment interruption related to electron and photon craniospinal irradiation in pediatric patients treated at the University of Texas M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2002; 52:1008–16.

6. Song KJ, Park JH, Im HJ, Ahn SD. Survival and long-term toxicities of pediatric Hodgkin lymphoma after combined modality treatment: a single institute experience. Radiat Oncol J. 2020; 38:198–206.

7. Lee J, Chung SY, Han JW, Kim DS, Kim J, Moon JY, et al. Treatment outcome of anaplastic ependymoma under the age of 3 treated by intensity-modulated radiotherapy. Radiat Oncol J. 2020; 38:26–34.

8. Barney CL, Brown AP, Grosshans DR, McAleer MF, de Groot JF, Puduvalli V, et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro Oncol. 2014; 16:303–9.

9. Brown AP, Barney CL, Grosshans DR, McAleer MF, de Groot JF, Puduvalli VK, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013; 86:277–84.

10. Yoon M, Shin DH, Kim J, Kim JW, Kim DW, Park SY, et al. Craniospinal irradiation techniques: a dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys. 2011; 81:637–46.

11. Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002; 54:824–9.

12. Ajithkumar T, Horan G, Padovani L, Thorp N, Timmermann B, Alapetite C, et al. SIOPE - Brain tumor group consensus guideline on craniospinal target volume delineation for high-precision radiotherapy. Radiother Oncol. 2018; 128:192–7.

13. Yoo GS, Yu JI, Cho S, Jung SH, Han Y, Park S, et al. Comparison of clinical outcomes between passive scattering versus pencil-beam scanning proton beam therapy for hepatocellular carcinoma. Radiother Oncol. 2020; 146:187–93.

14. Yu JI, Park HC, Yoo GS, Paik SW, Choi MS, Kim HS, et al. Clinical significance of systemic inflammation markers in newly diagnosed, previously untreated hepatocellular carcinoma. Cancers (Basel). 2020; 12:1300.

15. Martin AM, Raabe E, Eberhart C, Cohen KJ. Management of pediatric and adult patients with medulloblastoma. Curr Treat Options Oncol. 2014; 15:581–94.

16. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Yoo KH, et al. Induction chemotherapy reduces radiation therapy dose and volume in the treatment of intracranial germinoma: results of the SMC-G13 trial. Int J Radiat Oncol Biol Phys. 2020; 108:649–56.

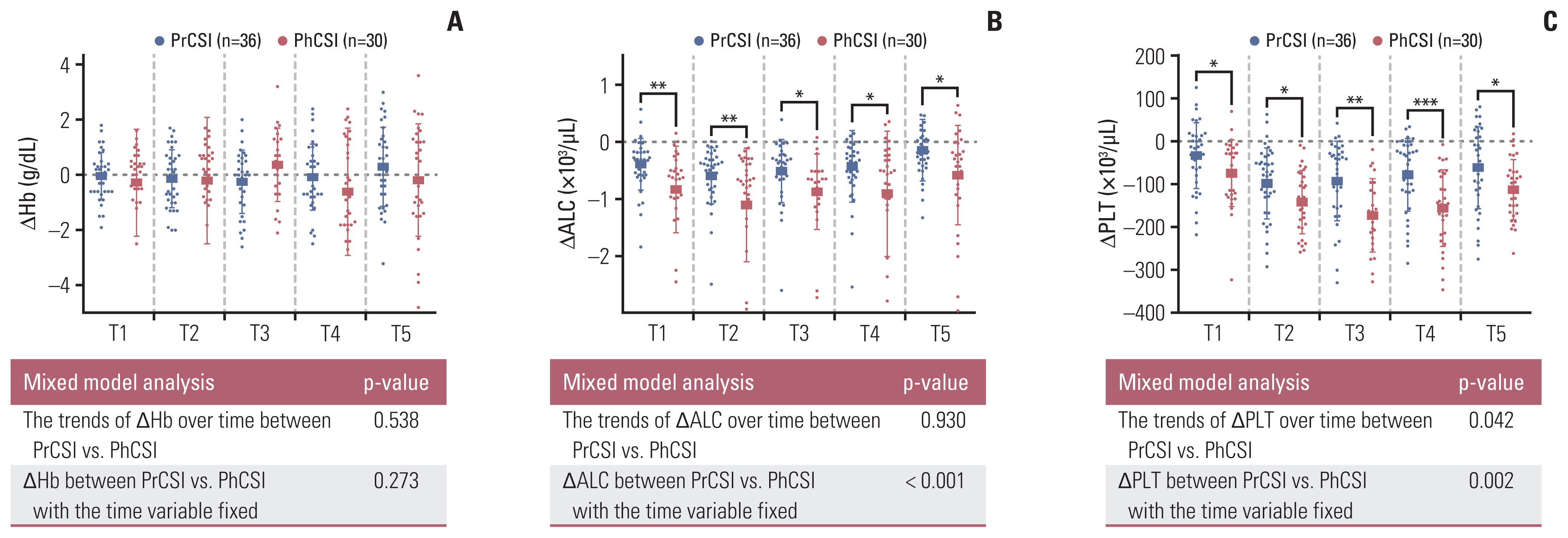
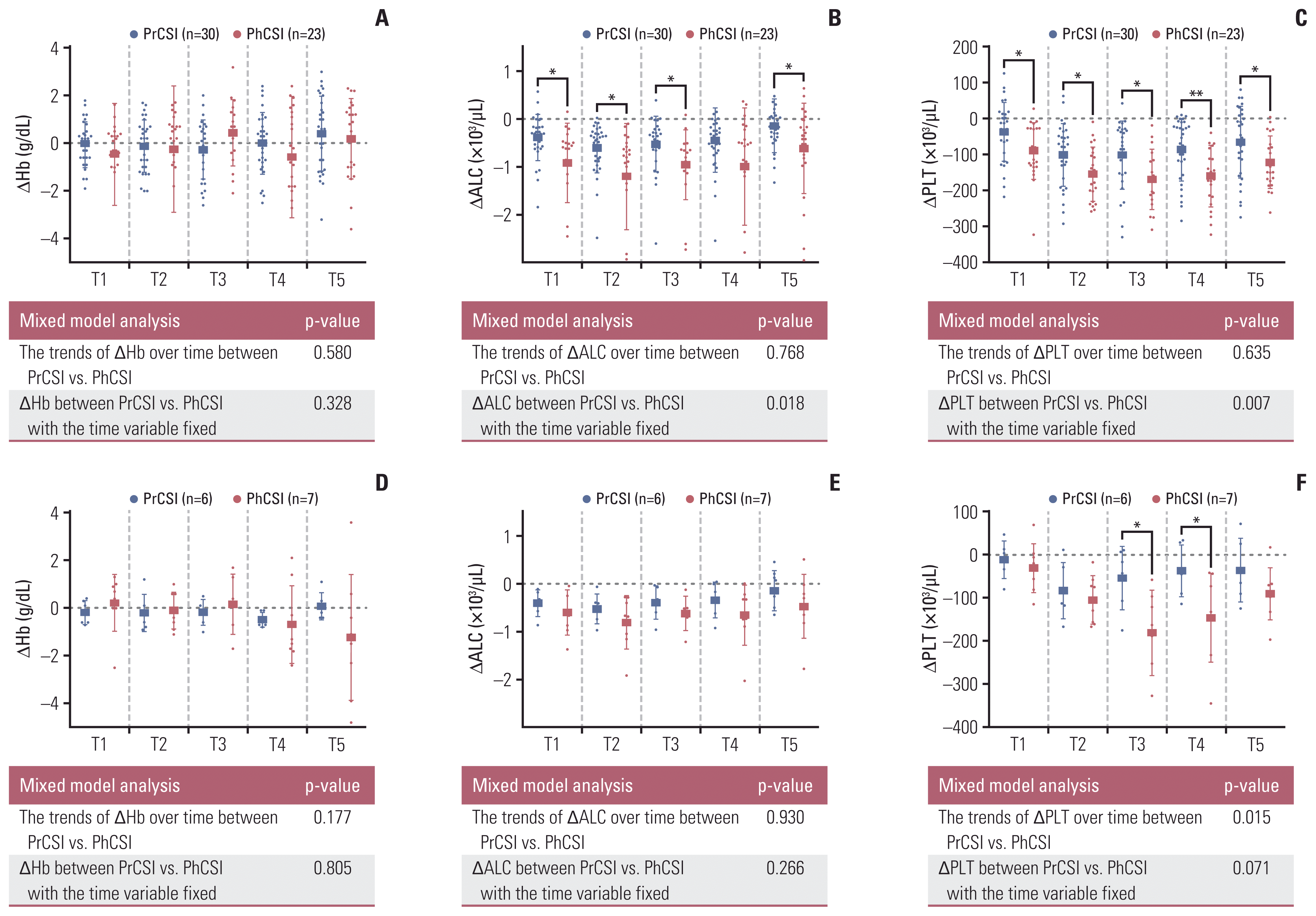
17. Deng W, Xu C, Liu A, van Rossum PS, Deng W, Liao Z, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019; 133:9–15.

18. Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, et al. Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: Immunologic relevance. Radiother Oncol. 2019; 131:52–9.

19. Hayman JA, Callahan JW, Herschtal A, Everitt S, Binns DS, Hicks RJ, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011; 79:847–52.

20. Hoeben BA, Carrie C, Timmermann B, Mandeville HC, Gandola L, Dieckmann K, et al. Management of vertebral radiotherapy dose in paediatric patients with cancer: consensus recommendations from the SIOPE radiotherapy working group. Lancet Oncol. 2019; 20:e155–66.

21. Paulino AC, Fowler BZ. Risk factors for scoliosis in children with neuroblastoma. Int J Radiat Oncol Biol Phys. 2005; 61:865–9.

22. MacEwan I, Chou B, Moretz J, Loredo L, Bush D, Slater JD. Effects of vertebral-body-sparing proton craniospinal irradiation on the spine of young pediatric patients with medulloblastoma. Adv Radiat Oncol. 2017; 2:220–7.

23. Jin JY, Mereniuk T, Yalamanchali A, Wang W, Machtay M, Spring Kong FM, et al. A framework for modeling radiation induced lymphopenia in radiotherapy. Radiother Oncol. 2020; 144:105–13.

24. So TH, Chan SK, Chan WL, Choi H, Chiang CL, Lee V, et al. Lymphopenia and radiation dose to circulating lymphocytes with neoadjuvant chemoradiation in esophageal squamous cell carcinoma. Adv Radiat Oncol. 2020; 5:880–8.

25. Xu C, Jin JY, Zhang M, Liu A, Wang J, Mohan R, et al. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol. 2020; 146:180–6.

26. Xie X, Lin SH, Welsh JW, Wei X, Jin H, Mohan R, et al. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung V5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother Oncol. 2021; 154:187–93.

27. Lambin P, Lieverse RIY, Eckert F, Marcus D, Oberije C, van der Wiel AM, et al. Lymphocyte-sparing radiotherapy: the rationale for protecting lymphocyte-rich organs when combining radiotherapy with immunotherapy. Semin Radiat Oncol. 2020; 30:187–93.

28. Ng SP, Bahig H, Jethanandani A, Pollard C, Berends J, Sturgis EM, et al. Lymphopenia during radiotherapy in patients with oropharyngeal cancer. Radiother Oncol. 2020; 145:95–100.

29. Miller JB, Figueroa EJ, Haug RM, Shah NL. Thrombocytopenia in chronic liver disease and the role of thrombopoietin agonists. Gastroenterol Hepatol (N Y). 2019; 15:326–32.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download