Abstract
Purpose
Children with Down syndrome (DS) show a higher risk of acute leukemia than those without DS. In this study, we investigated the nationwide incidence of acute leukemia among children with DS and compared their epidemiologic characteristics with those of children with acute leukemia but without DS.
Materials and Methods
Using the National Health Insurance Service database, we selected patients with acute leukemia aged 0–19 years at diagnosis between 2007 and 2016.
Results
Among the 4,697 children with acute leukemia, 54 (1.1%) had DS. The median incidence rate of leukemia with DS by year was 1.3% (range, 0.2% to 2.0%). Sixteen patients with acute lymphoblastic leukemia (ALL; 29.6%) and 36 with acute myeloid leukemia (AML; 66.7%) had DS. The DS group showed younger age at diagnosis than the non-DS group, and diagnosis of AML was more frequent in the DS group than in the non-DS group (3 years vs. 9 years, p < 0.001; 66.7% vs. 32.4%, p < 0.001, respectively). The 5-year overall survival was comparable between the DS and non-DS groups (88.0% vs. 81.9%, p=0.375). Among all the Koreans born between 2007 and 2008, the incidences of acute leukemia, ALL, and AML were 49.25, 20.75, and 163.38 times higher, respectively, in the DS group than in the non-DS group.
Down syndrome (DS) is the most common syndromic chromosomal abnormality in humans, occurring in 1 in 700 to 1,000 live births [1]. The association between DS and leukemia was first reported nearly 60 years ago, and several reports have also described an association between acute leukemia and DS [2–7]. The risk of acute leukemia for patients with DS has been reported to be 10 to 20 times higher than that for patients without DS [8]. It is estimated that acute megakaryocytic leukemia (AMKL) is particularly predominant among patients with DS, increasing the relative risk by 500 times compared to the general population [9]. Moreover, patients with DS who are younger than 5 years old develop acute myeloid leukemia (AML) more frequently than acute lymphoblastic leukemia (ALL) [10].
Previous studies have shown that AML with DS (DS AML) accounts for 6% to 14% of all pediatric AML cases, and that the treatment outcomes for DS AML are better than those for non-DS AML [11,12]. ALL with DS (DS ALL) accounts for about 2% of all pediatric ALL cases, and its treatment outcomes are worse than those for non-DS ALL [13,14]. However, since the prevalence of acute leukemia with DS is small, it is difficult to determine its exact incidence or survival rate. Moreover, there have been no reports of acute leukemia with DS in Korea.
Korea has a universal National Health Insurance Service (NHIS) system, which was launched by the government in 1989 and covers more than 97% of the population [15]. The NHIS database contains information such as details of diagnosis, prescription, and death for almost the entire population. The NHIS data is available for research purposes through a strict screening process, which allows for a more accurate approach to analysis of the incidences of rare diseases. Based on this background, we investigated the nationwide incidence of acute leukemia among children with DS using the NHIS database, and compared their epidemiologic characteristics and survival data with those of children with acute leukemia but without DS.
We assessed the NHIS database (NHIS-2019-1-612) and extracted data on patients with acute leukemia aged younger than 20 years old at diagnosis between 2006 and 2016. During this period, the number of new registered patients with acute leukemia with DS or without DS, their sex, age at diagnosis, and date of death were identified.
However, since the NHIS database captures the input from each medical institution, over-diagnosis is a likely occurrence. Moreover, acute leukemia or DS can be included in the NHIS database even if it was suspected but not confirmed. Therefore, we used the rare and intractable diseases registration (Rare Diseases Registry [RDR]) database, which was established with the NHIS in 2004 [16]. The RDR database involves the collation of data for each patient with a physician-certified diagnosis to provide accurate information. Therefore, we only selected the patients with acute leukemia with or without DS registered in the RDR database. Records from 2006 were screened to identify cases of patients previously diagnosed with acute leukemia. From January 2007 to December 2016, we collected the data of all patients aged 0 to 19 years old who were diagnosed with acute leukemia with or without DS in Korea. We also collected data on patients with acute leukemia with or without DS born from January 2007 to December 2008 to estimate the incidence of acute leukemia.
Cases with the International Classification of Disease 10th revision (ICD-10) codes Q90.0–Q90.2 and Q90.9 were defined as DS. Acute leukemia was defined as ALL (C91.0, C91.7, and C91.9), AML (C92.0, C92.3–C92.9, C93.0, C93.3–C93.9, C94.0, C94.2, and C94.7), and unspecified acute leukemia (C95.0, C95.7, and C95.9). RDR designated each disease with RDR-specific codes; V159 denotes DS, V011 indicates cases of pediatric cancer in patients aged below 18 years of age, V026 denotes leukemia, and V193 and V194 indicate registered patients with cancer. Data were collected only for patients with acute leukemia or DS with both ICD-10 codes and RDR-specific codes.
The probabilities of overall survival (OS) rate were estimated using the Kaplan-Meier method, and the survival curves were compared using the log-rank test. OS was defined as the time from diagnosis of acute leukemia to death from any cause. Patient characteristics were compared using Fisher exact test for binary variables and Mann-Whitney U test for continuous variables. Statistical differences were considered significant if p < 0.05. Data and statistical analyses were performed using SPSS ver. 19 (IBM Corp., Armonk, NY) and Prism ver. 7.04 (GraphPad Software, La Jolla, CA).
Based on the data extracted from the NHIS database, a total of 4,697 patients under the age of 20 years were diagnosed with acute leukemia from 2007 to 2016. ALL (n=2,758, 58.7%) was the most common disease, followed by AML (n=1,541, 32.8%) and unspecified acute leukemia (n=398, 8.5%). The median age at the time of diagnosis was 8 years old (range, 0 to 19 years). Of the 4,697 patients with acute leukemia, 54 (1.1%) had DS, of which 16 had DS ALL (0.6% of all patients with ALL), 36 had DS AML (2.3% of all patients with AML), and two had unspecified acute leukemia.
We divided the patients with acute leukemia into two groups according to diagnosis of DS, namely: acute leukemia with DS (n=54; 1.1%) and acute leukemia without DS (n=4,643, 98.9%). Table 1 shows the number of newly diagnosed leukemia patients with and without DS by year. The median incidence of leukemia with DS by year was 1.3%, with the lowest incidence in 2007 (0.2%) and the highest in 2008 (2.0%). Table 2 shows the patient characteristics of the two groups. AML was more frequent in the DS group than in the non-DS group (66.7% vs. 32.4%, p < 0.001). The median age at the time of diagnosis was younger in the DS group than in the non-DS group (3 years vs. 9 years, p < 0.001). In the DS group, 34 patients (63.0%) were younger than 5 years old and most of them had AML (n=29, 78.9%) (Fig. 1A). In the non-DS group, 1,342 patients (28.9%) were younger than 5 years old. ALL was the most common type of leukemia in the non-DS group (n=929, 69.2%) (Fig. 1B). The 5-year OS was similar between the DS and non-DS groups (88.0% vs. 81.9%, p=0.375) (Fig. 2A). The 5-year OS of ALL and AML was similar between the two groups as well (ALL, 88.2% vs. 85.6%, p=0.835; AML, 87.5% vs. 75.4%, p=0.114) (Fig. 2B and C).
The total number of births in Korea from 2007 to 2008 was 965,356, of which 397 children were diagnosed with DS. Like the patients with acute leukemia, we divided this population into a DS group (n=397) and a non-DS group (n=964,959). During the 10-year observation period, acute leukemia was diagnosed in 10 (2.5%, 10/397) and 506 (0.05%, 506/964,959) cases in the DS and non-DS groups, respectively. The relative risk of acute leukemia, ALL, and AML was 49.25, 20.75, and 163.38 times higher in the DS group than in the non-DS group, respectively (Table 3). Regarding all the patients with acute leukemia born between 2007 and 2008, the 5-year OS was comparable between the DS and non-DS groups (acute leukemia, 90.0% vs. 84.6%, p=0.619; ALL, 100% vs. 90.4%, p=0.569; AML, 83.3% vs. 68.9%, p=0.464, respectively).
In this study, acute leukemia with DS accounted 1.1% of all acute leukemia cases, DS AML accounted for 2.3% of all the AML cases, and DS ALL accounted for 0.6% of all the ALL cases. Compared to previous studies in which 6%–14% of children with AML and 2% of children with ALL had DS, our study revealed that a relatively small proportion of Korean children with acute leukemia have DS [11,12]. In the present study, the incidence of acute leukemia was higher in the DS group than in the general population, a finding that is in accordance with those of previous studies [8,10]. In a previous study, the proportion of women in the DS group was higher than that of men [12]. In the present study, the male to female ratio in the DS group was 1.8:1; however, there was no statistically significant difference between the DS group and the non-DS group. Moreover, our study results demonstrated that in the first 10 years of life, the incidence of acute leukemia was approximately 49-fold higher for patients in the DS group than for those in the non-DS group.
The exact mechanism by which trisomy 21 increases the incidence of leukemia has not been fully elucidated; however, this may be related to the gene dosage effect of the extra copy of chromosome 21 [17]. Mutations in several genes on chromosome 21 have been recognized in leukemia, many of which have been recognized as encoding transcription factors that act during various stages of hematopoiesis. RUNX1 (also known as AML1), a gene located on chromosome 21q22, is a transcription factor that regulates normal hematopoiesis. RUNX1 mutations are associated with 20% of pediatric ALL cases and 25% of pediatric AML cases [18]. It has been suggested that trisomy 21 causes GATA1 mutation, which may induce transient abnormal myelopoiesis and progression to myeloid leukemia, especially AMKL, in patients with DS [19]. Furthermore, exposure to environmental factors recognized as carcinogenic factors may increase the risk of developing leukemia in patients with DS [20]. It is necessary to clarify the mechanisms of leukemia in the future studies, and to develop a method that can effectively prevent the progression of leukemia in patients with DS.
The age distribution of the patients in our study demonstrated that AML is most common among patients with DS younger than 5 years old, a finding that is similar to those of previous studies [4,12]. In the present study, DS AML accounted for 2.3% of all the AML cases. This percentage is slightly smaller than those recorded in Western countries; 6% was recorded in a UK study [11] and 14% was recorded in a Nordic study [12]. It is related to the lower prevalence of DS in Korea than in Western countries [21]. The prevalence of DS among 0 to 19-year-old individuals is reported to be 16.2 per 100,000 in Korea [21] and 98.4 per 100,000 in the United Kingdom [22]. Moreover, this is also related to differences in the patient population between the previous studies and ours. As mentioned earlier, AML is more prevalent among patients with DS who are less 5 years old. Previous UK [11] and Nordic [12] studies included patients who were aged 0–14 years, whereas our study included patients who were aged 0–19 years. Therefore, we believe that the proportion of DS AML among the all the AML cases in this study was lower than that reported in previous studies.
DS AML has shown superior treatment outcomes than non-DS AML in previous studies [4,12]. A plausible reason for this could be the increased sensitivity of DS AML cells to cytotoxic drugs, especially cytarabine [23]. Moreover, differences in the expression of the gene encoding enzymes that metabolize cytotoxic drugs on chromosome 21 may explain the enhanced drug sensitivity of DS AML cells [24]. The results of the present study showed that the treatment outcome for AML was better in the DS group than in the non-DS group; however, there was no statistical difference between the two groups (87.5% vs. 75.4%, p=0.114). This may be associated with the improved treatment outcomes of the patients diagnosed with AML without DS in the present study compared to those of patients in previous studies [4,12]. However, since we only investigated OS as a treatment outcome, further research using data such as recurrence rates or events are necessary to generate more accurate results.
Generally, DS ALL rarely occurs in infancy [12,13]. No patients in the present study had DS ALL in infancy, a finding similar to those of previous studies. The treatment outcomes of patients with DS ALL have been reported to be inferior to those of patients with non-DS ALL [13,14]. This is because patients with DS ALL have a higher risk of induction failure than patients with non-DS ALL. This outcome is associated with the lower sensitivity of DS ALL cells to cytotoxic drugs [13,25]. Moreover, patients with DS ALL have higher treatment-related toxicities than patients with non-DS ALL [26]. This may be a poor prognostic factor for patients with DS ALL. However, the treatment results of DS ALL have recently improved, and the results of the present study showed comparable treatment outcomes between patients with DS ALL and patients with non-DS ALL. Our findings showed that the 5-year OS of patients with DS ALL was 88.2%, a percentage that is higher than the 75%–80% reported in some previous studies [13,27] but similar to that of a recent study which showed comparable treatment outcomes between patients with DS ALL and patients with non-DS ALL [28].
This study has some limitations. First, we identified 54 leukemia patients with DS in the NHIS and RDR databases; however, our study sample may not include patients with undiagnosed mosaic DS. Therefore, it is possible that the incidence of leukemia with DS was underestimated. Second, there were 396 patients (8.5%) with unspecified acute leukemia in the non-DS group who could be diagnosed with either ALL or AML. Therefore, the recorded percentage of patients with DS ALL and DS AML may be lower than the actual percentage. Third, as data were extracted from the NHIS and RDR databases, patient information such as data on cytogenetics, leukemia cell types, and treatment protocols were insufficient, therefore the factors related to the treatment outcomes could not be analyzed. Future nationwide multicenter studies conducted using detailed information of patients with DS and leukemia are necessary to enable the accurate identification of the clinical factors related to the prognosis of patients with DS. Despite these limitations, this is the first population-based study in Korea in which the epidemiology of acute leukemia with DS over a 10-year period was investigated using the NHIS database, and the survival rates of the DS and non-DS groups were compared. We also analyzed the relative risk of acute leukemia, ALL, and AML patients with DS compared with the general population.
In conclusion, we demonstrated that the incidence of acute leukemia among patients with DS is higher than that among those without DS. We also found that the OS rates are comparable between the DS and non-DS groups. Future nationwide multicenter studies are necessary to confirm the accurate incidence of acute leukemia and to determine the prognostic factors associated with leukemia with DS.
Notes
Ethical Statement
The study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2018-10-054). The need for informed consent was waived by the board.
ACKNOWLEDGMENTS
This study was supported by Samsung Medical Center grant (#SMO1190181). The funders had no role in study design, data collection and analysis, or preparation of the study.
References
1. Tabares-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011; 12:604–8.

2. Krivit W, Good RA. Simultaneous occurrence of mongolism and leukemia: report of a nationwide survey. AMA J Dis Child. 1957; 94:289–93.
3. Creutzig U, Ritter J, Vormoor J, Ludwig WD, Niemeyer C, Reinisch I, et al. Myelodysplasia and acute myelogenous leukemia in Down’s syndrome: a report of 40 children of the AML-BFM Study Group. Leukemia. 1996; 10:1677–86.
4. Lange BJ, Kobrinsky N, Barnard DR, Arthur DC, Buckley JD, Howells WB, et al. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children’s Cancer Group Studies 2861 and 2891. Blood. 1998; 91:608–15.
5. Kojima S, Sako M, Kato K, Hosoi G, Sato T, Ohara A, et al. An effective chemotherapeutic regimen for acute myeloid leukemia and myelodysplastic syndrome in children with Down’s syndrome. Leukemia. 2000; 14:786–91.

6. Lange B. The management of neoplastic disorders of haematopoiesis in children with Down’s syndrome. Br J Haematol. 2000; 110:512–24.
7. Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005; 19:1355–60.

8. Fong CT, Brodeur GM. Down’s syndrome and leukemia: epidemiology, genetics, cytogenetics and mechanisms of leukemogenesis. Cancer Genet Cytogenet. 1987; 28:55–76.

9. Hitzler JK, Zipursky A. Origins of leukaemia in children with Down syndrome. Nat Rev Cancer. 2005; 5:11–20.

10. Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down syndrome. Pediatr Blood Cancer. 2005; 44:8–12.

11. James R, Lightfoot T, Simpson J, Moorman AV, Roman E, Kinsey S, et al. Acute leukemia in children with Down’s syndrome: the importance of population based study. Haematologica. 2008; 93:1262–3.

12. Zeller B, Gustafsson G, Forestier E, Abrahamsson J, Clausen N, Heldrup J, et al. Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol. 2005; 128:797–804.

13. Buitenkamp TD, Izraeli S, Zimmermann M, Forestier E, Heerema NA, van den Heuvel-Eibrink MM, et al. Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood. 2014; 123:70–7.

14. Whitlock JA, Sather HN, Gaynon P, Robison LL, Wells RJ, Trigg M, et al. Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2005; 106:4043–9.

15. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014; 38:395–403.

16. Kim HS, Lyoo CH, Lee PH, Kim SJ, Park MY, Ma HI, et al. Current status of Huntington’s disease in Korea: a nationwide survey and national registry analysis. J Mov Disord. 2015; 8:14–20.

17. Xavier AC, Taub JW. Acute leukemia in children with Down syndrome. Haematologica. 2010; 95:1043–5.

18. Okuda T, Nishimura M, Nakao M, Fujita Y. RUNX1/AML1: a central player in hematopoiesis. Int J Hematol. 2001; 74:252–7.

19. Watanabe K. Recent advances in the understanding of transient abnormal myelopoiesis in Down syndrome. Pediatr Int. 2019; 61:222–9.

20. Mejia-Arangure JM, Fajardo-Gutierrez A, Flores-Aguilar H, Martinez-Garcia MC, Salamanca-Gomez F, Palma-Padilla V, et al. Environmental factors contributing to the development of childhood leukemia in children with Down’s syndrome. Leukemia. 2003; 17:1905–7.

21. Cho WK, Lee NY, Han K, Suh BK, Park YG. The population prevalence, associations of congenital heart defect and mortality risk for Down’s syndrome in South Korea based on National Health Insurance Service (NHIS) data. Clin Epidemiol. 2020; 12:519–25.
22. Alexander M, Ding Y, Foskett N, Petri H, Wandel C, Khwaja O. Population prevalence of Down’s syndrome in the United Kingdom. J Intellect Disabil Res. 2016; 60:874–8.

23. Zwaan CM, Kaspers GJ, Pieters R, Hahlen K, Janka-Schaub GE, van Zantwijk CH, et al. Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood. 2002; 99:245–51.

24. Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer. 2005; 44:33–9.

25. Frost BM, Gustafsson G, Larsson R, Nygren P, Lonnerholm G. Cellular cytotoxic drug sensitivity in children with acute leukemia and Down’s syndrome: an explanation to differences in clinical outcome? Leukemia. 2000; 14:943–4.

26. Pui CH, Raimondi SC, Borowitz MJ, Land VJ, Behm FG, Pullen DJ, et al. Immunophenotypes and karyotypes of leukemic cells in children with Down syndrome and acute lymphoblastic leukemia. J Clin Oncol. 1993; 11:1361–7.

Fig. 1
Age-specific distribution of patients diagnosed with acute leukemia from 2007 to 2016. (A) Acute leukemia with Down syndrome (DS). (B) Acute leukemia without DS. Among patients younger than 5 years of age, 63% of the patients in the DS group had acute myeloid leukemia (AML), and 69% of the patients in the non-DS group had acute lymphoblastic leukemia (ALL).
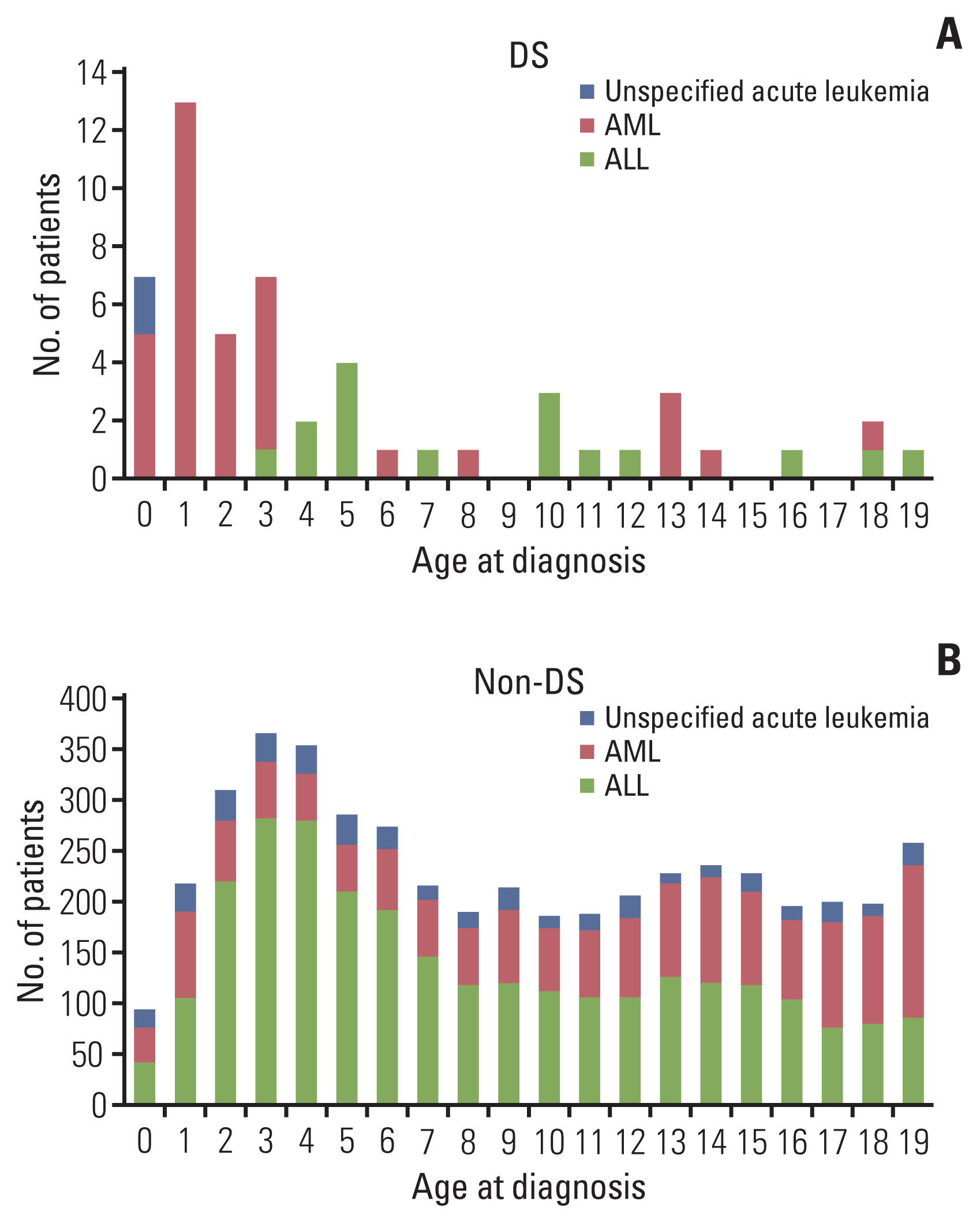
Fig. 2
Overall survival rates of patients with acute leukemia with or without Down syndrome (DS) from 2007 to 2016. (A) Acute leukemia. (B) Acute lymphoblastic leukemia (ALL). (C) Acute myeloid leukemia (AML). There was no statistical difference between the overall survival rates of patients with acute leukemia, ALL, and AML in the DS and non-DS groups.
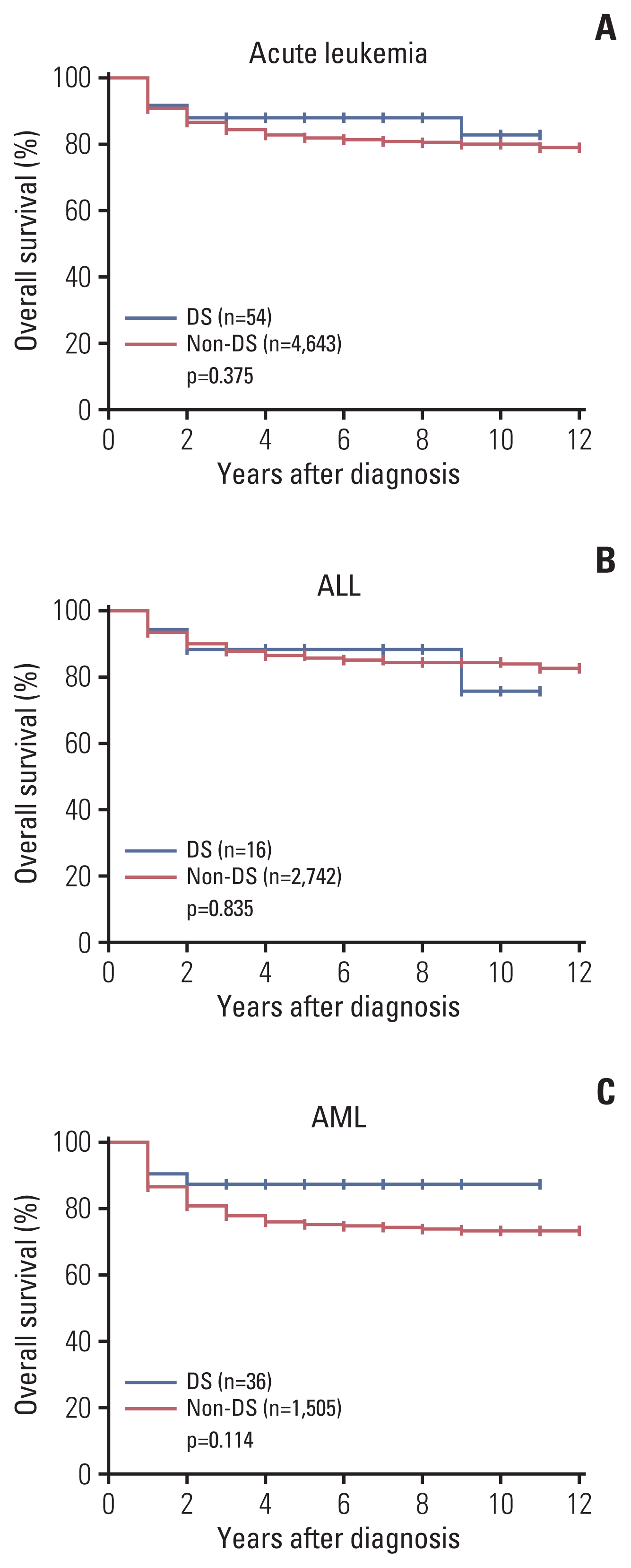
Table 1
Number of newly diagnosed patients with acute leukemia with or without DS by year in Korea
Table 2
Characteristics of patients diagnosed with acute leukemia with or without DS from 2007 to 2016
Table 3
Incidence of acute leukemia with or without DS among children born from 2007 to 2008




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download