Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
This study was approved by the Institutional Review Board of the Asan Medical Center (#2021-0111) and conducted in accordance with the Declaration of Helsinki. The IRB granted an informed-consent waiver for this retrospective study.
Author Contributions
Conceived and designed the analysis: Kim SB.
Collected the data: Jeong H.
Contributed data or analysis tools: Jeong JH, Kim JE, Ahn JH, Jung KH.
Performed the analysis: Jeong H.
Wrote the paper: Jeong H, Jeong JH.
Conflict of interest
KHJ reports personal fees from AstraZeneca, personal fees from Roche, personal fees from Celgene, personal fees from Novartis, personal fees from Takeda, outside the submitted work.
S-BK reports research funding from Novartis, Sanofi-Aventis and DongKook Pharm Co. and has participated as a consultant in advisory boards for Novartis, AstraZeneca, Lilly, Enzychem, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo, outside the submitted work. All remaining authors have declared no conflicts of interest.
References
Fig. 2
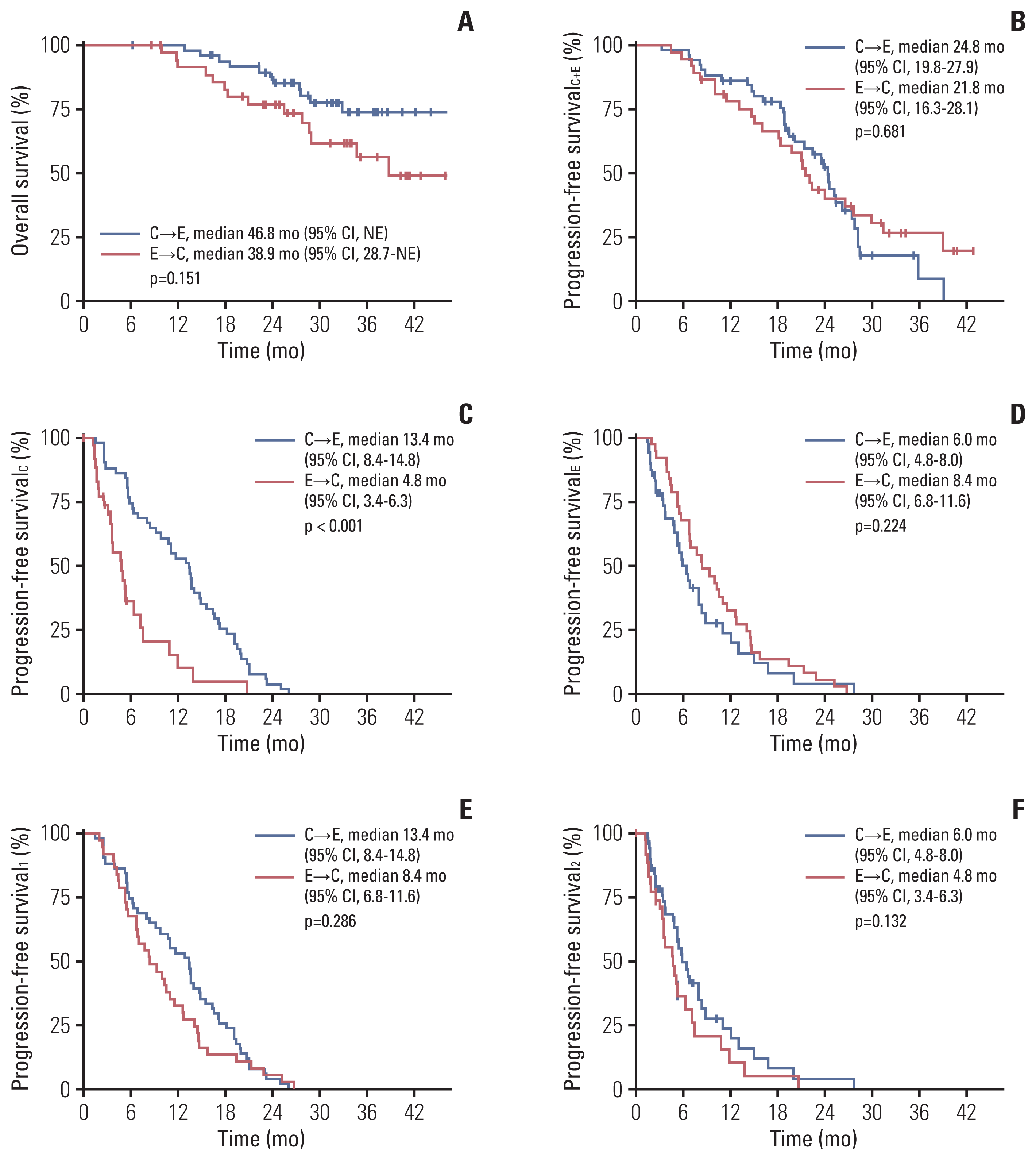
Fig. 3
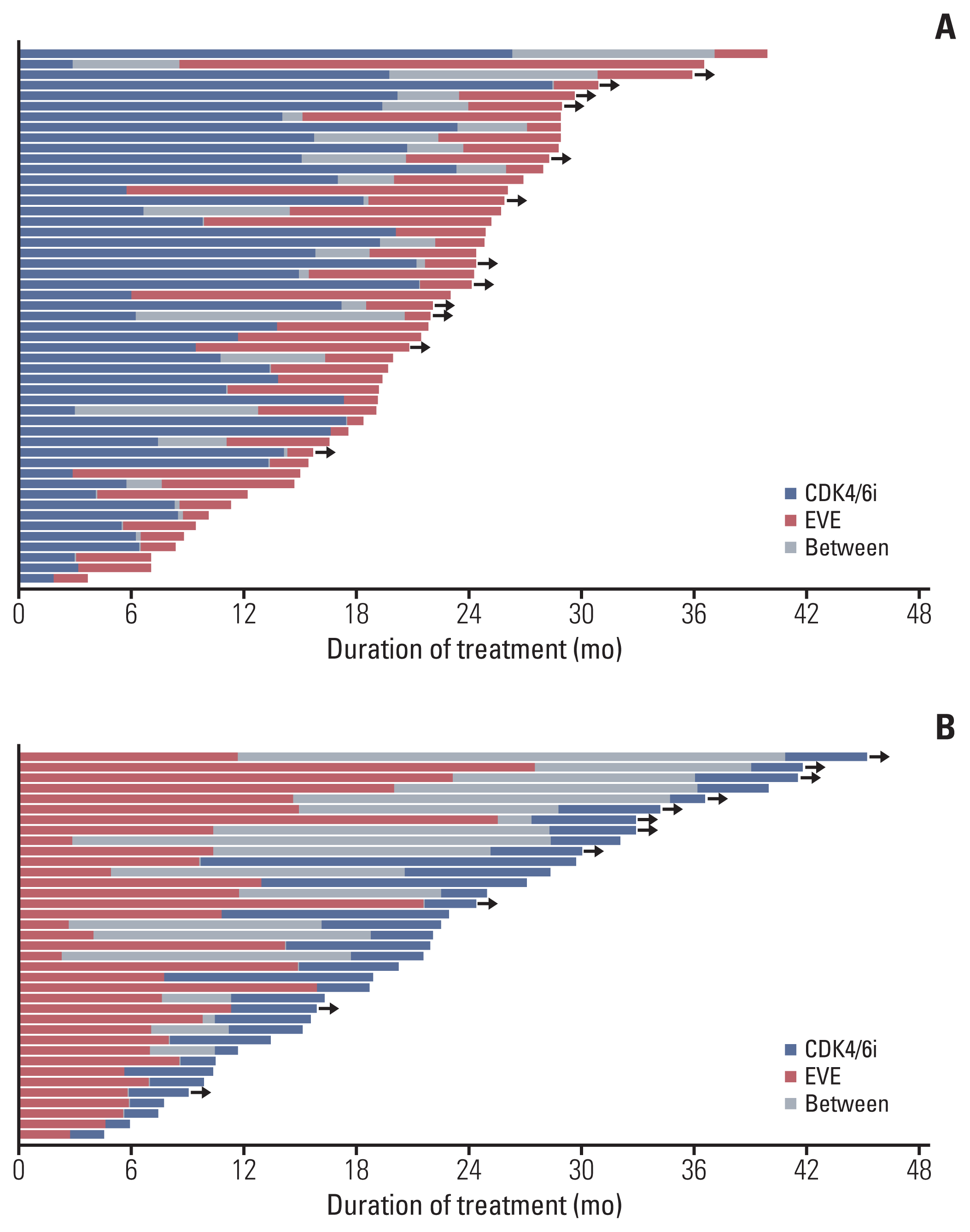
Table 1
| Overall (n=88) | C→E (n=51) | E→C (n=37) | p-value | |
|---|---|---|---|---|
| Age (yr) | 53 (35–76) | 52 (36–76) | 54 (35–70) | 0.568 |
| ECOG PS | ||||
| 0 | 19 (21.6) | 16 (31.4) | 3 (8.1) | 0.032 |
| 1 | 46 (52.3) | 23 (45.1) | 23 (62.2) | |
| Unknown | 23 (26.1) | 12 (23.5) | 11 (29.7) | |
| Menopausal status at diagnosis of advanced disease | ||||
| Pre/perimenopausal | 37 (42.0) | 21 (41.2) | 16 (43.2) | 0.916 |
| Postmenopausal | 49 (55.7) | 29 (56.9) | 20 (54.1) | |
| Unknown | 2 (2.3) | 1 (2.0) | 1 (2.7) | |
| Line of treatment | ||||
| 1 | 37 (42.0) | 33 (64.7) | 4 (10.8) | < 0.001 |
| 2 | 33 (37.5) | 15 (29.4) | 18 (48.6) | |
| ≥ 3 | 18 (20.5) | 3 (5.9) | 15 (40.5) | |
| Purpose of most recent endocrine treatment | ||||
| Adjuvant | 34 (38.6) | 30 (58.8) | 4 (10.8) | < 0.001 |
| Palliative | 46 (52.3) | 13 (25.5) | 33 (89.2) | |
| No prior endocrine treatment | 8 (9.1) | 8 (15.7) | 0 | |
| Previous palliative endocrine treatment | 46 | 13 | 33 | |
| Tamoxifen only | 1 (2.2) | 1 (7.7) | 0 | 0.167 |
| Letrozole | 23 (50.0) | 7 (53.8) | 16 (48.5) | |
| Tamoxifen and letrozole | 18 (39.1) | 3 (23.1) | 15 (45.5) | |
| Others | 4 (8.7) | 2 (15.4) | 2 (6.1) | |
| Disease status | ||||
| Initially metastatic | 22 (25.0) | 13 (25.5) | 9 (24.3) | > 0.99 |
| Recurrent | 66 (75.0) | 38 (74.5) | 28 (75.7) | |
| Disease-free intervala) | 66 | 38 | 28 | |
| ≤ 24 mo | 9 (13.6) | 5 (13.2) | 4 (14.3) | > 0.99 |
| > 24 mo | 57 (86.4) | 33 (86.8) | 24 (85.7) | |
| Previous endocrine resistanceb) | ||||
| Endocrine-sensitive | 58 (65.9) | 36 (70.6) | 22 (59.5) | 0.030 |
| Endocrine-resistant | 22 (25.0) | 7 (13.7) | 15 (40.5) | |
| No endocrine treatment | 8 (9.1) | 8 (15.7) | 0 | |
| Previous palliative chemotherapy | ||||
| Exposed | 19 (21.6) | 4 (7.8) | 15 (40.5) | 0.001 |
| Unexposed | 69 (78.4) | 47 (92.2) | 22 (59.5) | |
| ER/PR status | ||||
| ER+ | 88 (100) | 51 (100) | 37 (100) | - |
| PR+ | 54 (61.4) | 32 (62.7) | 22 (59.5) | 0.928 |
| No. of metastatic sites | ||||
| 1 | 29 (33.0) | 17 (33.3) | 12 (32.4) | 0.663 |
| 2 | 30 (34.1) | 19 (37.3) | 11 (29.7) | |
| ≥ 3 | 29 (33.0) | 15 (29.4) | 14 (37.8) | |
| Sites of metastases | ||||
| Bone | 63 (71.6) | 34 (66.7) | 29 (78.4) | 0.335 |
| Bone only | 15 (17.0) | 8 (15.7) | 7 (18.9) | 0.912 |
| Visceral | 66 (75.0) | 40 (78.4) | 26 (70.3) | 0.533 |
| Treatment regimen | ||||
| Everolimus+exemestane | 88 (100) | 51 (100) | 37 (100) | - |
| Palbociclib+letrozole | 40 (45.5) | 40 (78.4) | 0 | < 0.001 |
| Palbociclib+fulvestrant | 47 (53.4) | 11 (21.6) | 36 (97.3) | |
| Abemaciclib+fulvestrant | 1 (1.1) | 0 | 1 (2.7) | |
Values are presented as median (range) or number (%). C, cyclin-dependent kinase 4/6 inhibitor; E, everolimus; ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; PR, progesterone receptor.
Table 2
| C→E | E→C | p-value | |
|---|---|---|---|
| Best overall response to CDK4/6 inhibitor-based regimen | Measurable (n=37) | Measurable (n=32) | |
| Partial response | 18 (48.6) | 7 (21.9) | |
| Stable disease | 15 (40.5) | 13 (40.6) | |
| Progressive disease | 4 (10.8) | 11 (34.4) | |
| Not evaluated | 0 | 1 (3.1)a) | |
| Best overall response to EVE-based regimen | Measurable (n=44) | Measurable (n=30) | |
| Partial response | 4 (9.1) | 6 (20.0) | |
| Stable disease | 28 (63.6) | 20 (66.7) | |
| Progressive disease | 10 (22.7) | 4 (13.3) | |
| Not evaluated | 2 (4.5) | 0 | |
| Overall response rateb) | |||
| Overall response rate to EVE | 4/42 (9.5) | 6/30 (20.0) | 0.302 |
| Overall response rate to CDK4/6 inhibitor | 18/37 (48.6) | 7/31 (22.6) | 0.049 |




 PDF
PDF Citation
Citation Print
Print


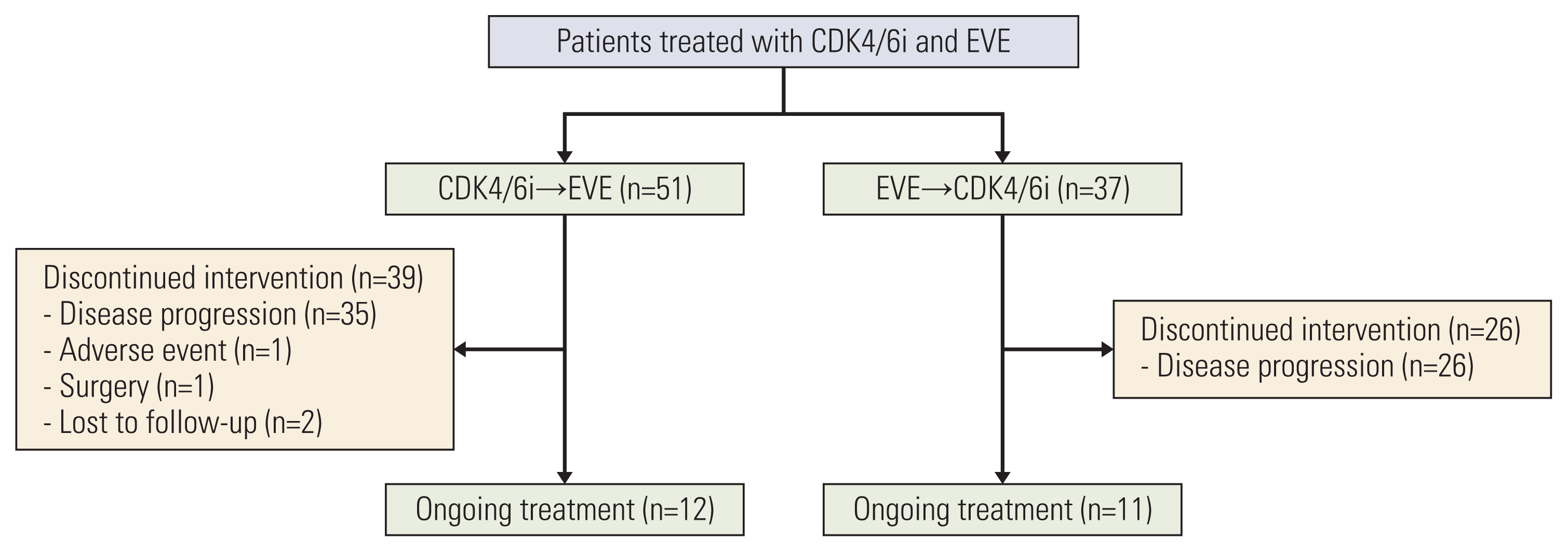
 XML Download
XML Download