Introduction
Sarcoma is a group of disorder containing various histologic types of tumors, which arise from mesenchymal tissue. It is largely divided into bone and soft tissue sarcoma. Malignant bone and soft tissue sarcoma account for 6% and 7% of all cancers occurring under age 20, respectively [
1,
2].
Outcome of sarcoma has improved significantly during the last several decades, and the adjusted 5-year survival rates for both bone and soft tissue in the United States and Korea were reported to be 71%–77% [
3,
4]. However, recurrent or refractory patients fare much less, with 5-year overall survival (OS) rate of < 40% [
3]. Attempts have been made to obtain a better survival for relapsed or refractory sarcoma. Several salvage regimens, including the combination of ifosfamide, carboplatin, and etoposide (ICE) [
5]; topotecan and cyclophosphamide (TC) [
6]; docetaxel and gemcitabine [
7]; irinotecan and temozolomide with vincristine (VIT) or without vincristine (IT) [
8,
9] have been employed. Several studies testing VIT have revealed an enthusiastic response rate of 29%–63% in recurrent or refractory Ewing sarcoma [
8–
10]. However, reports on VIT trial are restricted mostly to Ewing sarcoma and rhabdomyosarcoma of pediatric age. Under this background, we wanted to explore the usefulness of the VIT regimen in children and young adults with various sarcoma histology.
Irinotecan is a camptothecin prodrug that is converted into SN-38
in vivo and acts as a topoisomerase I poison. Topoisomerase I inhibitors stabilize the enzyme-DNA covalent complex and cause S-phase specific cytotoxicity. Temozolomide is an oral alkylating agent. DNA damage from alkylators increases the sensitivity to topoisomerase I inhibitors. Schedule-dependent synergy between temozolomide and irinotecan has been shown in preclinical studies when temozolomide was given at least 1 hour before irinotecan administration [
11]. Another advantage of these two-drug combination is that they have non-overlapping toxicities and differing mechanisms of resistance [
12]. In addition, vincristine is known to show synergistic effect with irinotecan and temozolomide in rhabdomyosarcoma and Ewing sarcoma [
13].
Herein, we describe the antitumor response, survival outcome, and toxicity experienced with VIT retrospectively collected in our patients with relapsed or refractory sarcoma.
Go to :

Discussion
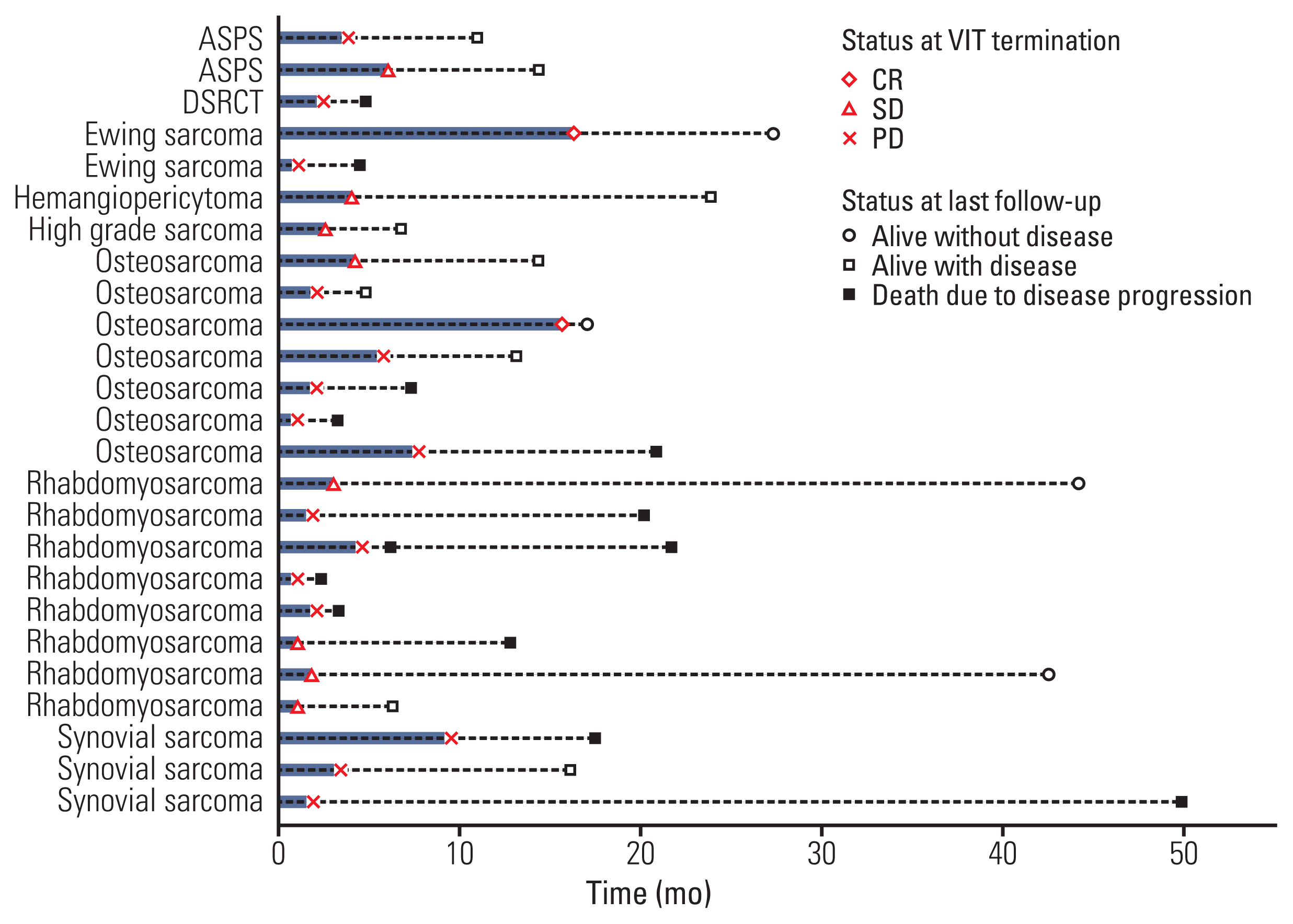
Herein, we demonstrate that VIT administered as a salvage regimen was effective in tumor control and tolerable in various types of relapsed or refractory sarcomas. Although the number of subjects in each disease type was quite small, VIT achieved more than 50% control rate. VIT trials in other studies have been attempted mostly in rhabdomyosarcoma and Ewing sarcoma [
11,
12,
16]. Only anecdotal data testing VIT (or IT) in osteosarcoma, synovial sarcoma, alveolar soft part sarcoma, and desmoplastic small round cell tumor are available. Furthermore, we could not find any VIT data on hemangiopericytoma, and mixed rhabdomyosarcoma and liposarcoma. SD was noted in two diseases thus, we could raise the possibility that VIT application might be expanded to more diverse sarcoma types than the ones ever reported. One (50%) of the two patients with Ewing sarcoma and one (14.3%) of the seven patients with osteosarcoma showed PR. Although exempted from response evaluation due to lack of visible tumor (excision of metastatic lung tumor before starting VIT), the Ewing sarcoma patient described above had been heavily pretreated with four regimens and two times of pulmonary metastatectomy before he finally received VIT. Thus, it is possible to speculate that VIT might have contributed to his survival without disease over 6 years, considering that two times of previous pulmonary metastatectomy alone had not eradicated the disease. Benefit of VIT in Ewing sarcoma has been demonstrated in other studies as well [
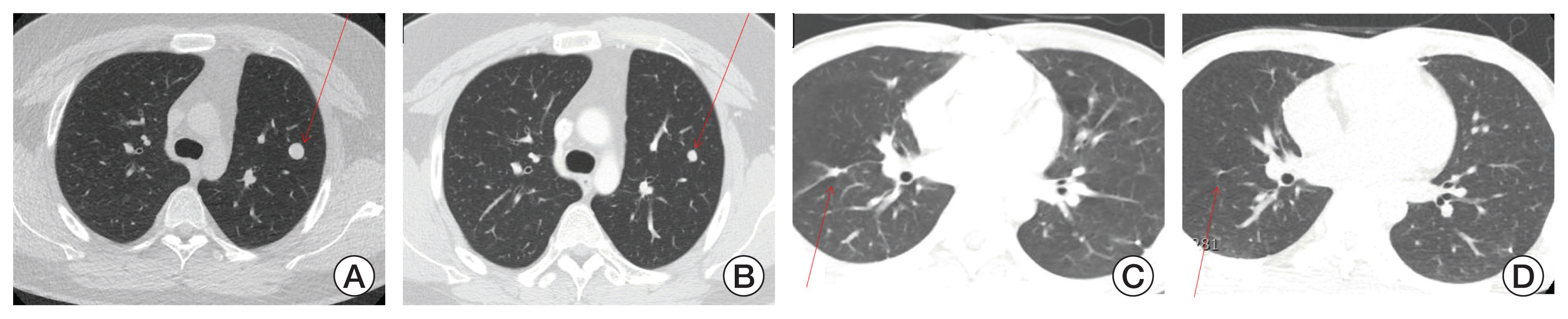
11]. Interestingly, the patient with rhabdomyosarcoma who displayed discrepancy between radiologic image and histologic findings described earlier is alive without disease for more than 3.5 years off-therapy. Clinical variables, such as disease type, number of recurrences before VIT treatment, and sites of tumor at the beginning of VIT could not predict VIT response.
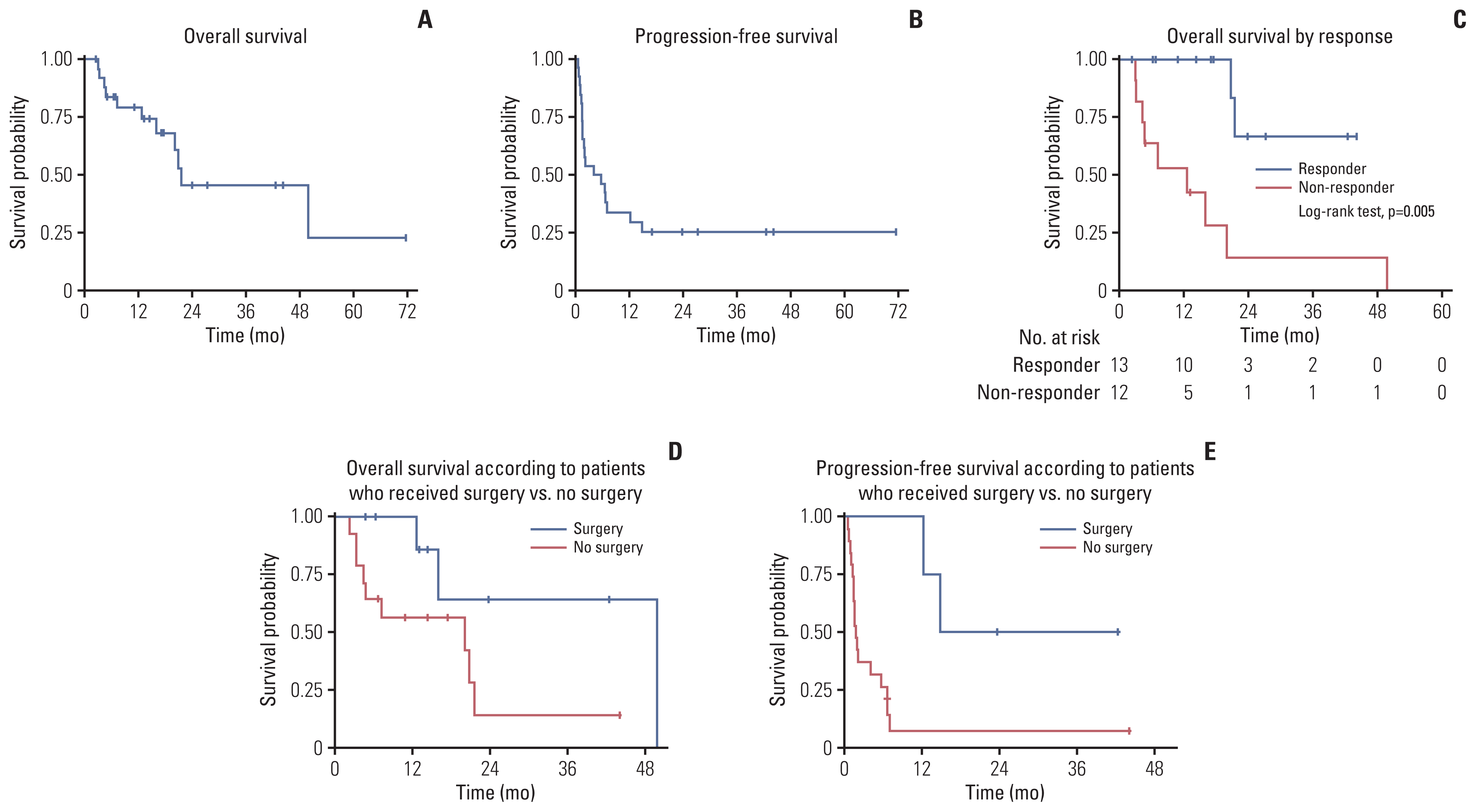
OS at 1 year and 2 years were 79.3% (95% CI, 57.1 to 90.9) and 45.5% (95% CI, 20.6 to 67.5), respectively, and PFS at 1 year and two years were 33.9% (95% CI, 16.7 to 52.0) and 25.4% (95% CI, 10.6 to 43.3), respectively (
Fig. 3A and B). One-year and two-year OS in Ewing sarcoma patients were 55% [
17] and 25.9% [
10] in other series. PFS varied from 23% to 49% depending on the studies [
16,
17]. However, comparison on outcomes between our study and other series is not plausible as patient population was quite different.
Most of the patients in our study were heavily pretreated, and VIT was delivered as a median of 4th line (range, 2 to 7). Our median value of VIT line was greater than that of any other studies employing irinotecan and temozolomide with or without vincristine in sarcomas [
9,
12,
16,
17]. In fact, VIT was adopted as 3rd and 4th line in our patients with PR. This suggests that certain proportion of sarcoma patients with multiple relapses or primary refractoriness still have a chance for prolonged survival or even cure, even though VIT is administered after several times of treatment failure.
Clinical variables, such as sex, disease type, number of relapses or progression before starting VIT, number of previous regimens, and the presence of metastases at the start of VIT did not predict outcome. On the contrary, VIT response was associated with outcome, which is consistent with the report on Ewing sarcoma [
17]. Besides VIT response, beneficial effect of surgery on survival was examined in our subjects, excluding two patients with PR on VIT from analysis. Patients who had surgical resection fare better in terms of survival. (
Fig. 3D and E) This suggests that surgery needs to be considered whenever possible even in patients who do not respond to VIT.
In previous studies, irinotecan and temozolomide with or without vincristine have been shown to be effective most commonly in Ewing sarcoma [
8–
10,
12,
17,
18]. In agreement with this, PR was observed in one of the two evaluable patients with Ewing sarcoma in our series. In addition, we raise the possibility that VIT was effective in the non-evaluable patient with Ewing sarcoma who underwent VIT after metastectomy and maintained long-term CR afterwards. Another PR was seen in one of the seven patients with osteosarcoma. Studies on VIT activity in osteosarcoma are quite limited, and objective response was reported in the range between 25% (one of the four patients) and 50% (one of the two patients) [
8,
19,
20]. Although objective response rate in our osteosarcoma patients was 14.2%, it is hard to make any comparison since number of subjects in the literature is too small.
In the current study, we included patients with synovial sarcoma, alveolar soft part sarcoma, hemangiopericytoma, desmoplastic small round cell tumor, and mixed rhabdomyosarcoma and liposarcoma. Interestingly, SD was observed in all of those with exception of desmoplastic small round cell tumor. This suggests that VIT might be useful across various sarcoma histology. VIT trial for synovial sarcoma [
20] and desmoplastic small round cell tumor [
19] is available in only a couple of studies, and that for hemangiopericytoma and mixed rhabdomyosarcoma and liposarcoma has not been reported yet.
As was predicted, gastrointestinal toxicities were most common. Grade 2 diarrhea occurred in 30.8% of the patients, although cefixime was prescribed prophylactically. However, it was manageable with loperamide with or without atropine prescription. With regards to grade 3 or higher toxicity, grade 3 nausea and/or vomiting, grade 3 colitis, grade 3 neutropenic fever, and grade 4 thrombocytopenia was demonstrated in one patient (3.8%) for each. There was no life-threatening morbidity or treatment-related mortality. In a study on Ewing sarcoma [
10], grade 3 or higher diarrhea occurred in 13.6%, and grade 3 or higher neutropenia and thrombocytopenia occurred in 4.5% for each. In another study on Ewing sarcoma [
17], grade 3 or higher diarrhea, neutropenia, thrombocytopenia, and nausea and/or vomiting developed in 4%, 12%, 4%, and 2%, respectively. Compared with these studies, incidence rate of grade 3 or higher toxicities was comparable; however, ≥ grade 3 diarrhea was not reported in our series. Vigorous prescription of antidiarrheals with prophylactic cefixime might have played a role.
Our results have some limitations. First, it is a retrospective analysis based on small sample size. Second, incidence of actual toxicities could have been reported less as data were collected retrospectively. However, we believe that at least grade 3 or higher toxicities were not likely to be missed.
The current study has several implications. First, various sarcoma histology, such as rhabdomyosarcoma, osteosarcoma, Ewing sarcoma, synovial sarcoma, alveolar soft part sarcoma, hemangiopericytoma, desmoplastic small round cell tumor, and mixed rhabdomyosarcoma and liposarcoma were included in the study. To our knowledge, there has been no report on VIT trial for hemangiopericytoma and mixed rhabdomyosarcoma and liposarcoma, and only a couple of reports for synovial sarcoma and alveolar soft part sarcoma. In addition, SD was observed in these four sarcoma types, suggesting that VIT as a palliative regimen might be utilized broadly across various sarcoma histology. Second, VIT was administered in heavily pretreated patients considering that our median VIT line was fourth, the highest value ever reported. Nonetheless, we could obtain 52% control rate. Moreover, the regimen was tolerable enough that only one of the 26 patients refused to take the regimen in the middle due to grade 3 nausea and vomiting. One plausible reason for low toxicity is low hematologic toxicity of VIT regimen. Taken these together, we argue that VIT may remain an option even when there is little hope for further treatment in a far advanced sarcoma.
Conclusively, we demonstrate that the VIT regimen was active and tolerable in our cohort with a variety of relapsed and/or refractory sarcoma. However, even with our meaningful VIT response, long-term survival is still not satisfactory. Therefore, further efforts need to be pursed such as, VIT combined with targeted or immune-oncologic agents at least in certain histologic types of sarcoma.
In conclusion, with the VIT regimen delivered in 26 recurrent and/or refractory patients with various sarcoma histology, we could achieve 8% PR and 52% control rate. OS and PFS rates were 79.3% and 33.9% at 1 year, and 45.5% and 25.4% at 2 years, respectively. Even though the regimen was given at a late stage of treatment with a median line of 4th, it was quite tolerable. Grade 3 and grade 4 toxicity was observed in 11.5% and 3.8% of our cohort, respectively, and serious treatment-related morbidity or mortality did not occur. Thus, the VIT regimen was active and tolerable in our recurrent and/or refractory sarcoma patients with various histology.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download