Abstract
Purpose
Materials and Methods
Results
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of the National Cancer Center and Cancer Research Institute in Korea (IRB no. NCC2015-0217). Obtaining informed consent from the study participants was waived as this study used deidentified administrative data.
Author Contributions
Conceived and designed the analysis: Yang MS, Park M, Lee GH, Shin JH, Kim YA.
Collected the data: Yang MS, Back JH, Kim YA.
Contributed data or analysis tools: Yang MS, Back JH.
Performed the analysis: Yang MS.
Wrote the paper: Yang MS, Park M, Kim YA.
Critical revision of the manuscript for important intellectual content: Yang MS, Park M, Back JH, Lee GH, Shin JH, Kim K, Seo HJ, Kim YA.
Supervision: Kim YA.
ACKNOWLEDGMENTS
References
Fig. 1
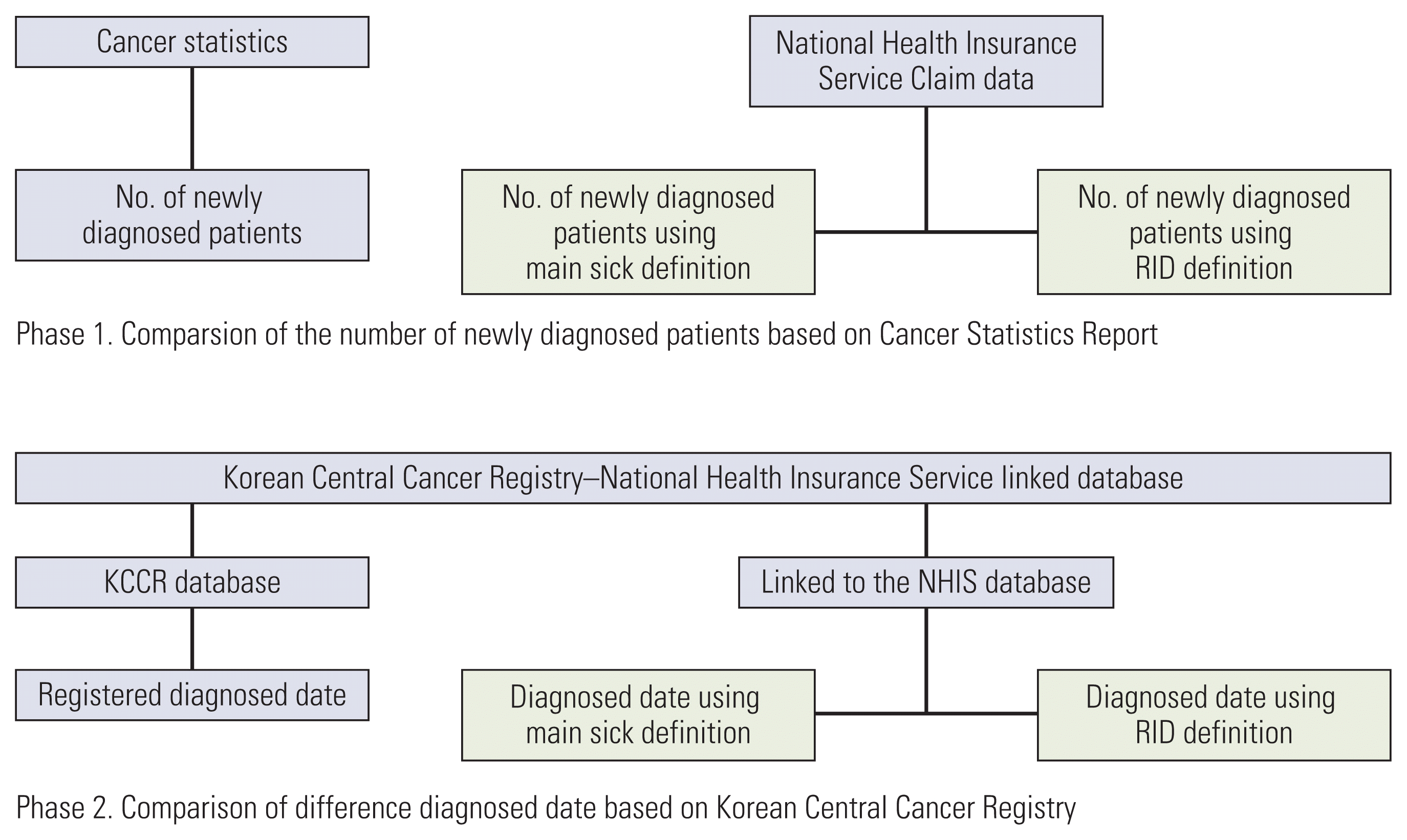
Fig. 2
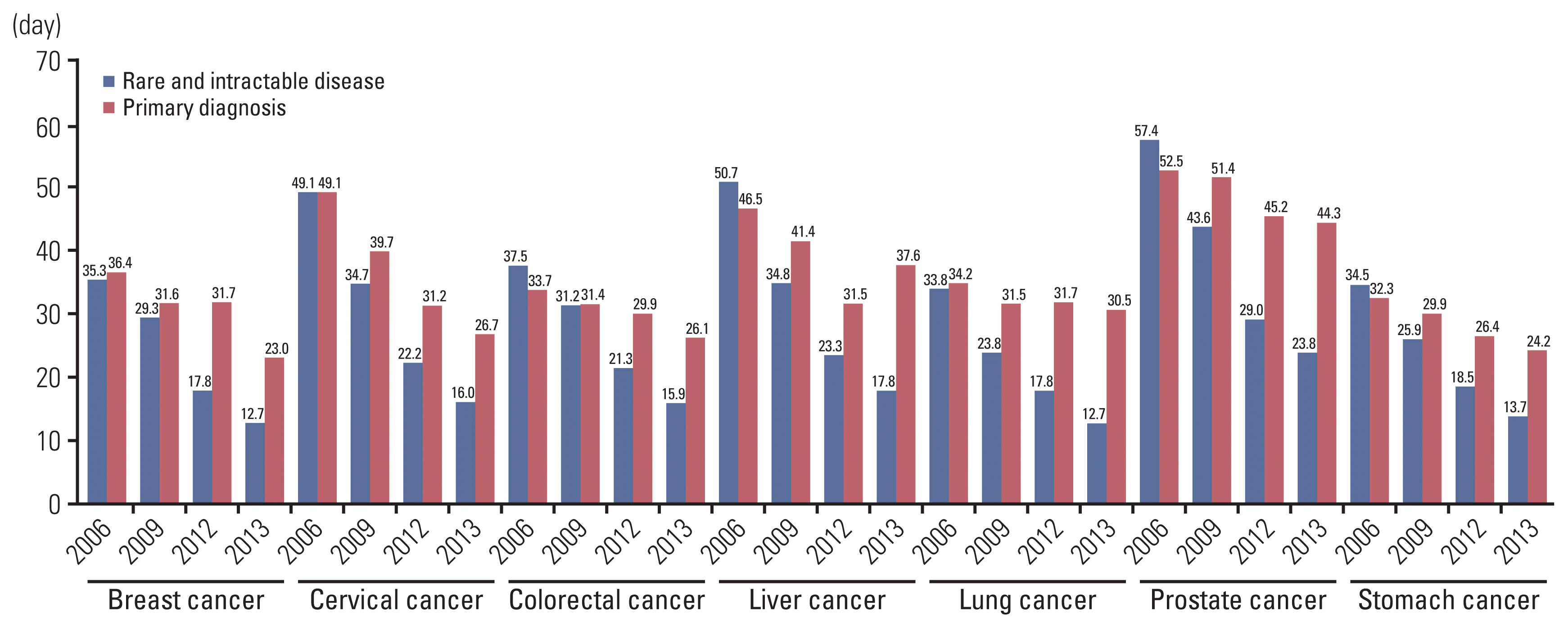
Fig. 3
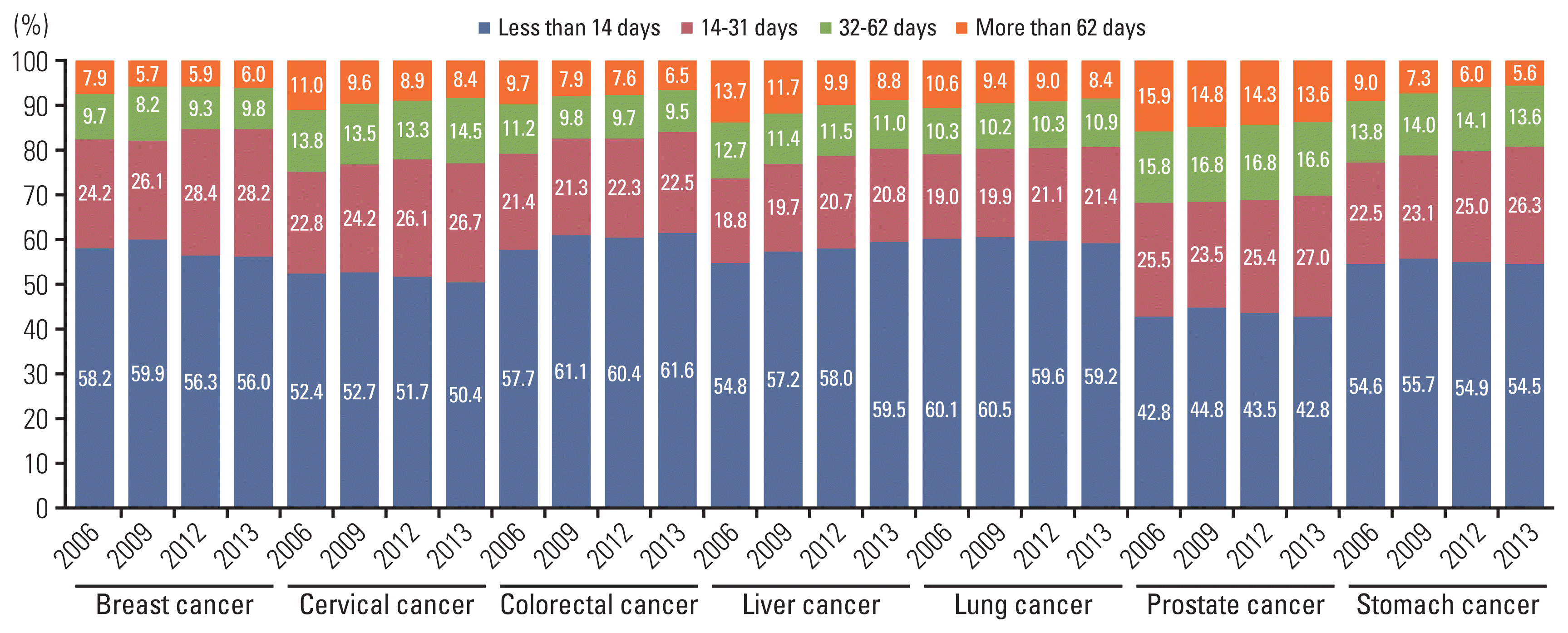
Fig. 4
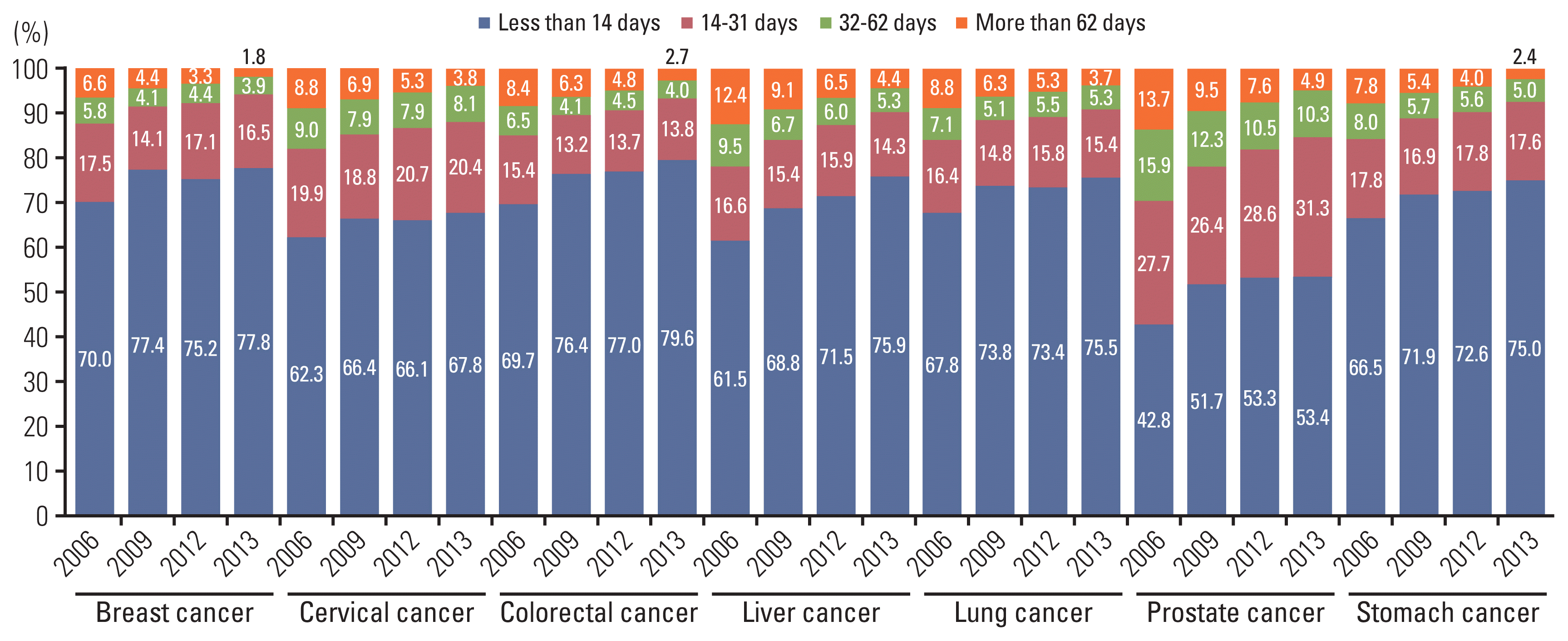
Table 1
| Cancer type | Year | Cancer statistics (A) | Primary diagnosis(B)a) (B-A) | RID(C)b) (C-A) |
|---|---|---|---|---|
| Stomach | 2006 | 26,460 | 28,746 (990) | 29,303 (306) |
| 2009 | 30,040 | 31,030 (967) | 31,336 (124) | |
| 2012 | 31,133 | 32,100 (690) | 31,976 (409) | |
| 2015 | 29,337 | 30,027 (1,530) | 30,436 (1,052) | |
| Liver | 2006 | 14,970 | 16,500 (1,074) | 15,448 (135) |
| 2009 | 16,006 | 17,080 (2,151) | 16,945 (887) | |
| 2012 | 16,130 | 18,281 (2,025) | 17,394 (360) | |
| 2015 | 15,874 | 17,899 (818) | 18,259 (536) | |
| Colorectal | 2006 | 19,920 | 20,738 (645) | 21,274 (706) |
| 2009 | 25,520 | 24,875 (168) | 25,581 (81) | |
| 2012 | 29,497 | 29,329 (690) | 29,410 (552) | |
| 2015 | 27,043 | 27,733 (1,137) | 28,285 (1,973) | |
| Lung | 2006 | 17,741 | 18,878 (258) | 16,905 (938) |
| 2009 | 20,086 | 20,344 (1,217) | 19,406 (1,216) | |
| 2012 | 22,526 | 23,743 (825) | 22,527 (548) | |
| 2015 | 24,502 | 25,327 (1,612) | 24,779 (874) | |
| Breast | 2006 | 10,951 | 12,563 (1,536) | 13,437 (536) |
| 2009 | 13,693 | 15,229 (1,248) | 15,765 (222) | |
| 2012 | 16,784 | 18,032 (1,532) | 18,254 (674) | |
| 2015 | 19,301 | 20,833 (1,053) | 21,507 (1,132) | |
| Cervical | 2006 | 4,064 | 5,117 (599) | 6,249 (619) |
| 2009 | 3,832 | 4,431 (685) | 5,050 (218) | |
| 2012 | 3,664 | 4,349 (649) | 4,567 (391) | |
| 2015 | 3,616 | 4,265 (704) | 4,656 (332) | |
| Prostate | 2006 | 4,527 | 5,231 (562) | 4,899 (327) |
| 2009 | 7,533 | 8,095 (641) | 7,768 (385) | |
| 2012 | 9,393 | 10,034 (597) | 9,649 (302) | |
| 2015 | 10,304 | 28,746 (990) | 29,303 (306) |
Table 2
| Cancer type | Method | Sensitivity (95% CI) | Positive predictive value (95% CI) |
|---|---|---|---|
| Stomach | Primary diagnosisa) | 96.0 (96.0–96.1) | 94.1 (94.0–94.2) |
| RIDb) | 95.7 (95.7–95.8) | 93.9 (93.8–94.0) | |
| Liver | Primary diagnosis | 92.2 (92.0–92.3) | 85.6 (85.4–85.8) |
| RID | 91.9 (91.7–92.0) | 86.0 (85.8–86.1) | |
| Colorectal | Primary diagnosis | 91.5 (91.4–91.7) | 92.4 (92.2–92.5) |
| RID | 92.3 (92.2–92.4) | 91.8 (91.6–91.9) | |
| Lung | Primary diagnosis | 95.0 (94.8–95.1) | 88.9 (88.8–89.1) |
| RID | 93.1 (93.0–93.3) | 90.2 (90.1–90.4) | |
| Breast | Primary diagnosis | 97.9 (97.8–98.0) | 91.4 (91.3–91.6) |
| RID | 98.1 (98.0–98.2) | 89.6 (89.4–89.7) | |
| Cervical | Primary diagnosis | 93.8 (93.6–94.1) | 81.8 (81.3–82.2) |
| RID | 94.4 (94.1–94.7) | 76.3 (75.9–76.8) | |
| Prostate | Primary diagnosis | 94.7 (94.5–94.9) | 91.9 (91.7–92.1) |
| RID | 95.3 (95.1–95.5) | 93.7 (93.5–93.9) |
Table 3
| Cancer type | Year | KCCR data | Primary diagnosisa) | RIDb) |
|---|---|---|---|---|
| Stomach | 2006 | 25,614 | 22,612 (88.3) | 22,696 (88.6) |
| 2009 | 29,408 | 26,122 (88.8) | 26,704 (90.8) | |
| 2012 | 30,616 | 27,213 (88.9) | 27,848 (91.0) | |
| 2013 | 29,906 | 26,692 (89.3) | 27,441 (91.8) | |
| Liver | 2006 | 14,191 | 11,722 (82.6) | 11,514 (81.1) |
| 2009 | 15,479 | 13,044 (84.3) | 13,279 (86.0) | |
| 2012 | 15,882 | 13,595 (85.6) | 14,066 (88.6) | |
| 2013 | 15,839 | 13,803 (87.2) | 14,283 (90.2) | |
| Colorectal | 2006 | 19,298 | 16,568 (85.9) | 16,754 (86.8) |
| 2009 | 24,930 | 21,172 (84.9) | 21,791 (87.4) | |
| 2012 | 28,832 | 24,584 (85.3) | 25,233 (87.5) | |
| 2013 | 27,321 | 23,791 (87.1) | 24,229 (89.4) | |
| Lung | 2006 | 16,414 | 14,286 (87.0) | 13,865 (84.5) |
| 2009 | 18,928 | 16,673 (88.1) | 16,816 (88.8) | |
| 2012 | 21,301 | 19,000 (89.2) | 19,303 (90.6) | |
| 2013 | 22,413 | 20,136 (89.8) | 20,470 (91.3) | |
| Breast | 2006 | 10,802 | 9,769 (90.4) | 9,913 (91.8) |
| 2009 | 13,547 | 12,345 (91.1) | 12,620 (93.2) | |
| 2012 | 16,586 | 15,184 (91.6) | 15,560 (93.8) | |
| 2013 | 17,229 | 15,900 (92.3) | 16,370 (95.0) | |
| Cervical/Uterine | 2006 | 3,956 | 3,370 (85.2) | 3,439 (86.9) |
| 2009 | 3,742 | 3,170 (84.7) | 3,282 (87.7) | |
| 2012 | 3,574 | 3,064 (85.7) | 3,160 (88.4) | |
| 2013 | 3,599 | 3,191 (88.7) | 3,266 (90.8) | |
| Prostate | 2006 | 4,406 | 3,585 (81.4) | 3,660 (83.1) |
| 2009 | 7,397 | 6,277 (84.9) | 6,488 (87.7) | |
| 2012 | 9,242 | 7,896 (85.4) | 8,305 (89.9) | |
| 2013 | 9,454 | 8,195 (86.7) | 8,598 (91.0) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download