Abstract
Background
Mechanically ventilated patients experience anxiety for many reasons. Pharmacological treatments such as benzodiazepines are commonly employed to manage anxiety; however, these therapies often cause undesired side effects. Additional therapies for anxiety management are needed. We sought to determine whether cell phone-based virtual reality therapy could feasibly be used for anxiety management in mechanically ventilated patients.
Methods
Mechanically ventilated subjects underwent at least one session of virtual reality therapy in which they were shown a cinematic video of an outdoor green space or blue space with 360° visual range of motion. Goal session duration was 5 minutes. The primary outcome was incidence of predefined patient safety events, including self-extubation and accidental removal of tubes or lines.
Results
Ten subjects underwent a total of 18 virtual reality sessions. Fifteen sessions lasted the planned 5 minutes, one session was extended at participant request, and two sessions were terminated early at participant request. There were no occurrences of the predefined safety events, and no occurrences of cybersickness. Use of a visual analog scale to measure anxiety level was feasible for this pilot study, demonstrating feasibility of this scale for future, larger scale studies.
Mechanically ventilated patients experience acute anxiety due to dyspnea, uncomfortable stimuli such as endotracheal tubes, feelings of loss of control, and fears related to their underlying disease. Current anxiety therapies rely almost solely on pharmacotherapies such as benzodiazepines, which carry undesirable side effects and increase the risk of development of delirium [1]. Additionally, survivors of critical illness show increased incidence of chronic depression and anxiety disorders [2,3], and exposure to mechanical ventilation [4], and benzodiazepines [5] have been shown to be risk factors.
While some adjuncts such as music interventions have been explored [6-8], there remain few non-pharmacologic options for anxiety control. Over the last decade, virtual reality (VR) technology has improved in quality, decreased in cost, and become more accessible. Application of VR technology reduces perceived pain and anxiety in patients undergoing painful procedures [9-14], and it has been successfully used to promote cognitive stimulation in critically ill patients [15]. Despite these advances, VR use for mechanically ventilated patients carries safety risks such as accidental dislodgement of lines or endotracheal tubes, and cybersickness, a motion sickness-like syndrome which can occur with VR exposure consisting of symptoms of headache, nausea, vomiting, dizziness, and gastric fullness [16]. We explored the feasibility of VR for anxiolysis in mechanically ventilated patients using a single-arm prospective trial.
The study protocol was approved by the local Institutional Review Board prior to initiation, and this trial is logged at clinicaltrials.gov registry number NCT03169374. Requests for sharing of de-identified individual patient data will be honored within the constraints of the local IRB’s data sharing policies; this process can be initiated by contacting the corresponding author. Written informed consent was obtained from all study participants.
Per our inclusion and exclusion criteria each participant consented for themselves; consent from legally authorized representatives was not permitted. Participants posed any questions to investigators by writing, and all questions were answered. The consenting process concluded with a brief series of seven yes/no questions asked of the participant to ensure comprehension of the study’s purpose and protocols.
Two intensive care units (ICUs), one dedicated medical ICU at an academic center and one general ICU at a community hospital, were screened at the investigators’ convenience for patients on mechanical ventilation. Inclusion criteria were patients of age 18 years or more, on mechanical ventilation, able to maintain a spontaneous wakeful state and able to communicate with researchers. Exclusion criteria were expected liberation from the ventilator within 12 hours, delirium (as assessed by routine confusion assessment method for the intensive care unit testing), inability of the subject to consent for themselves, history of cybersickness or pre-existing symptoms of cybersickness, skull injury or surgery precluding use of the VR visor head strap, auditory or visual impairment, positive end expiratory pressure ventilator setting greater than 10 cm H2O, inability to safely remove patient restraints for VR sessions, known difficult airway, or tracheostomy placed within the previous seven days (Figure 1). Our initial enrollment goal was 30 subjects, based on guidance from the United States Food and Drug Administration for traditional feasibility studies [17].
Enrolled patients underwent at least one session of VR therapy with a goal duration of 5 minutes. This short duration was chosen to minimize the risk of cybersickness, which increases with duration of VR use [18]. Cinematic VR, in which VR is presented as 360° video via a head-mounted display (HMD), was generated using a Samsung Galaxy S7 smartphone (Samsung, Seoul, Korea) coupled with low-cost headsets (Topmaxions; Wuzhong Zhongtai Youlian Technology & Trade, Wuzhong, China), which housed the smartphone to create the HMD. Participants were issued a single headset upon enrollment to the trial, used this headset for all trial VR sessions, and were allowed to keep their headset on trial completion. Sound was provided via the smart phone speakers, rather than employing headphones. Participants were shown relaxing environments using the 360° video feature of the video streaming service YouTube. The videos used for this purpose can be found on the YouTube channel “ICU virtual reality” (https://www.youtube.com/channel/UCTi6OIJkVzLk1PFi-cNPXpg). Environments consisted of various outdoor green and blue spaces, which were chosen for their positive impact on affect in prior research [19]. A single stationary camera position was used for each environment to minimize motion-induced cybersickness and to facilitate gentle, small angle head movements to explore the environment [20]. Videos played in real time via the YouTube application. Subjects were able to examine environments through gentle head movement within the constraints of ventilator hosing and other external tethers, but could not interact with any elements within the environments. While complete range of head movement is not possible with ventilated participants, the advantage of using VR over a two-dimensional monoscopic display is that the ICU environment is occluded while wearing the HMD facilitating immersion in an alternate, virtual world of nature scenes.
The primary outcome was incidence of the predefined patient safety events listed in Table 1. This collection of events has been previously determined in the physical therapy literature to be of particular concern when providing therapy to ICU patients [21]. Although VR does not involve the same vigorous movements as physical therapy, it does involve head movement and some degree of active patient participation. We therefore felt these events to be similarly important for monitoring in initial VR sessions as part of the feasibility pilot. Secondary outcomes were session duration, number of sessions per day, number of days each participant undergoes at least one session, reason for termination of sessions terminated before 5 minutes, measurement of blood pressure, pulse and respiratory rate at beginning and end of each session, incidence of cybersickness, and change in subjective anxiety levels with VR intervention as measured using the 100-point visual analog scale-anxiety (VAS-A), which has previously been validated as an assessment of anxiety levels [22] and used in studies of music interventions for mechanically ventilated patients [8]. Change in anxiety level was included as a secondary outcome to ensure feasibility of using the scoring system in this setting and for use in a larger scale efficacy trial. Standard descriptive and comparative statistical analyses were conducted on the collected primary and secondary outcomes data to ensure feasibility of this approach for a larger study. For measurements of anxiety and vital sign parameters, the differences between starting and ending values for each session (paired differences) were compared to the null set using the Wilcoxon signed-rank test. This test was used because some variables were non-normally distributed.
We screened 81 mechanically ventilated patients between November 2017 and June 2019. Fifteen of these were eligible to participate in the study and ten consented to do so (Figure 1). Of screened patients who were not eligible, inability to maintain a wakeful state or to communicate with researchers were the chief reasons for not meeting criteria. Baseline characteristics of the ten participants are shown in Table 2.
Results of our primary and secondary outcomes are listed in Table 1. A total of 18 VR sessions were performed for the ten participants; two participants had a single session, and eight participants had two sessions, each performed on separate days. Prespecified safety and cybersickness outcomes were successfully monitored according to protocol as part of feasibility. We observed no incidence of the prespecified safety outcomes, including accidental dislodgment of an endotracheal tube or tracheostomy. Additionally, no participant reported symptoms of cybersickness. Use of the 100-point VAS-A scale was feasible in this setting and showed a modest median decrease of 8.5 points following VR sessions. No participant completed more than one session per day. Five additional sessions had been planned but were not carried out for reasons listed in Table 2. Fifteen sessions lasted the planned duration of 5 minutes, and one lasted 15 minutes at participant request. Two sessions were terminated early at participant request, one because the participant felt homesick, the other participant would not give a reason.
Although our initial goal was 30, we closed the study after enrollment of ten subjects due to low rates of accrual. This was largely driven by a low prevalence of mechanically ventilated patients who were able to remain wakeful and adequately communicate with researchers to provide consent for the study and indicate anxiety levels (Figure 1).
As VR technology continues to improve, it has been increasingly utilized in the medical setting to promote patient comfort. Feasibility and effectiveness of VR interventions in the ICU setting have been explored using healthy volunteers emulating ICU patients [23,24], and using actual ICU patients who are not intubated or otherwise mechanically ventilated [25]. Additionally, use of an interactive computer program which senses patient motion as input and provides visual output through a flat panel video screen has been used to provide neurocognitive stimulation in both intubated and non-intubated patients; however this system was not fully immersive as it did not employ HMDs [15].
Use of HMDs to provide fully immersive VR for mechanically ventilated patients carries unique challenges, including the risk of accidental dislodgement of the endotracheal tube or tracheal cannula and associated sequelae [26,27] due to increased head movements, and reciprocally the possibility of reduction in enjoyability of VR sessions due to head motion restrictions imposed by ventilator hosing. Furthermore, the communication limitations imposed by mechanical ventilation can limit assessment of subjective outcomes such as pain and anxiety. This has limited the use of fully immersive VR in mechanically ventilated patients.
Although our study only comprised 10 participants undergoing 18 VR sessions, we successfully monitored prespecified safety and cybersickness outcomes, neither of which occurred during this feasibility study. Additionally, we were able to effectively utilize a 100-point VAS-A to assess patient anxiety levels before and after sessions of VR, with a median paired decrease of 8.5 points associated with VR intervention (P=0.012). While this suggests feasibility of VAS-A use in future VR studies, the low sample size and novel implementation of this pilot study prohibits any conclusions about VR effectiveness.
A major barrier to the use of VR broadly among mechanically ventilated patients was inability of patients to maintain a spontaneously wakeful state. Likely due to sedative medications and effects of underlying critical illness, the majority of patients we screened for this study were unable to meet this criterion, and we ultimately closed our enrollment prematurely because of this. More flexible study protocols, for example allowing the controlled reduction of sedatives to enable participation, may improve recruitment in future studies, but this would need to be balanced against possible undue discomfort to patients, and for the present study we did not feel this was justified.
As a feasibility pilot project, our study is limited by its small size and single-arm design. Although we did not have any safety events occur during our study, its limited size prevents us from establishing a rate at which these events will occur in a larger population. In addition, while we noted a modest decrease in subjective anxiety levels following VR sessions, interpretation of this is not warranted given the pilot nature of this study, and we certainly cannot establish a causal relationship due to the absence of a control group. Finally, enrollment was hindered by the fact that many mechanically ventilated patients were not conscious or communicative enough to participate; future studies will need to take this constraint into consideration. Despite these limitations, we feel that VR technology shows potential for anxiety management in mechanically ventilated patients as demonstrated by the implementation of this feasibility study and warrants further rigorous exploration.
▪ A pilot design of a cinematic virtual reality environment to relieve anxiety was implemented according to protocol for a pilot cohort of mechanically ventilated patients without incidence of prespecified safety outcomes including accidental extubation, and without incidence of cybersickness.
▪ A 100-point visual analog scale was successfully applied to assess subjects’ anxiety levels before and after virtual reality interventions, demonstrating feasibility of this scale for future studies.
ACKNOWLEDGMENTS
This project was funded by the University of Minnesota Critical Care Program and was supported by the University of Minnesota Clinical and Translational Science Institute (National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494).
We thank Dr. Craig Weinert for critically reading this manuscript.
REFERENCES
1. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007; 298:2644–53.

2. Ludmir J, Netzer G. Go big: measuring and tackling psychological morbidity after critical illness. Ann Am Thorac Soc. 2016; 13:1217–8.

3. Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016; 43:23–29.

4. Wunsch H, Christiansen CF, Johansen MB, Olsen M, Ali N, Angus DC, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014; 311:1133–42.

5. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015; May. 43:1121–9.
6. Lee CH, Lee CY, Hsu MY, Lai CL, Sung YH, Lin CY, et al. Effects of music intervention on state anxiety and physiological indices in patients undergoing mechanical ventilation in the intensive care unit. Biol Res Nurs. 2017; 19:137–44.

7. Mofredj A, Alaya S, Tassaioust K, Bahloul H, Mrabet A. Music therapy, a review of the potential therapeutic benefits for the critically ill. J Crit Care. 2016; 35:195–9.

8. Chlan LL, Weinert CR, Heiderscheit A, Tracy MF, Skaar DJ, Guttormson JL, et al. Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: a randomized clinical trial. JAMA. 2013; 309:2335–44.

9. Faber AW, Patterson DR, Bremer M. Repeated use of immersive virtual reality therapy to control pain during wound dressing changes in pediatric and adult burn patients. J Burn Care Res. 2013; 34:563–8.

10. Markus LA, Willems KE, Maruna CC, Schmitz CL, Pellino TA, Wish JR, et al. Virtual reality: feasibility of implementation in a regional burn center. Burns. 2009; 35:967–9.

11. Small C, Stone R, Pilsbury J, Bowden M, Bion J. Virtual restorative environment therapy as an adjunct to pain control during burn dressing changes: study protocol for a randomised controlled trial. Trials. 2015; 16:329.

12. Nilsson S, Finnström B, Kokinsky E, Enskär K. The use of virtual reality for needle-related procedural pain and distress in children and adolescents in a paediatric oncology unit. Eur J Oncol Nurs. 2009; 13:102–9.

13. Mosso-Vázquez JL, Gao K, Wiederhold BK, Wiederhold MD. Virtual reality for pain management in cardiac surgery. Cyberpsychol Behav Soc Netw. 2014; 17:371–8.

14. Dascal J, Reid M, IsHak WW, Spiegel B, Recacho J, Rosen B, et al. Virtual reality and medical inpatients: a systematic review of randomized, controlled trials. Innov Clin Neurosci. 2017; 14:14–21.
15. Turon M, Fernandez-Gonzalo S, Jodar M, Gomà G, Montanya J, Hernando D, et al. Feasibility and safety of virtual-reality-based early neurocognitive stimulation in critically ill patients. Ann Intensive Care. 2017; 7:81.

16. Weech S, Kenny S, Barnett-Cowan M. Presence and cybersickness in virtual reality are negatively related: a review. Front Psychol. 2019; 10:158.

17. Hoffmann MJ. FDA regulatory process. Silver Spring (MD): U.S. Food and Drug Administration;2021. [cited 2021 July 7]. Available from: https://www.fda.gov/media/90419/download.
18. Rebenitsch L, Owen C. Review on cybersickness in applications and visual displays. Virtual Real. 2016; 20:101–25.

19. McMahan EA, Estes D. The effect of contact with natural environments on positive and negative affect: a meta-analysis. J Posit Psychol. 2015; 10:507–19.

20. Bruck S, Watters PA. Accessible virtual reality therapy using portable media devices. Stud Health Technol Inform. 2010; 154:87–91.
21. Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014; 29:395–400.

22. Chlan LL. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs. 2004; 48:493–9.

23. Gerber SM, Jeitziner MM, Wyss P, Chesham A, Urwyler P, Müri RM, et al. Visuo-acoustic stimulation that helps you to relax: a virtual reality setup for patients in the intensive care unit. Sci Rep. 2017; 7:13228.

24. Gerber SM, Jeitziner MM, Sänger SD, Knobel SEJ, Marchal-Crespo L, Müri RM, et al. Comparing the relaxing effects of different virtual reality environments in the intensive care unit: observational study. JMIR Perioper Med. 2019; 2:e15579.

25. Ong TL, Ruppert MM, Akbar M, Rashidi P, Ozrazgat-Baslanti T, Bihorac A, et al. Improving the intensive care patient experience with virtual reality-a feasibility study. Crit Care Explor. 2020; 2:e0122.

Figure 1.
Flow diagram outlining screening and enrollment of participants, as well as inclusion and exclusion criteria. CAM-ICU: confusion assessment method for the intensive care unit; VR: virtual reality; PEEP: positive end-expiratory pressure.
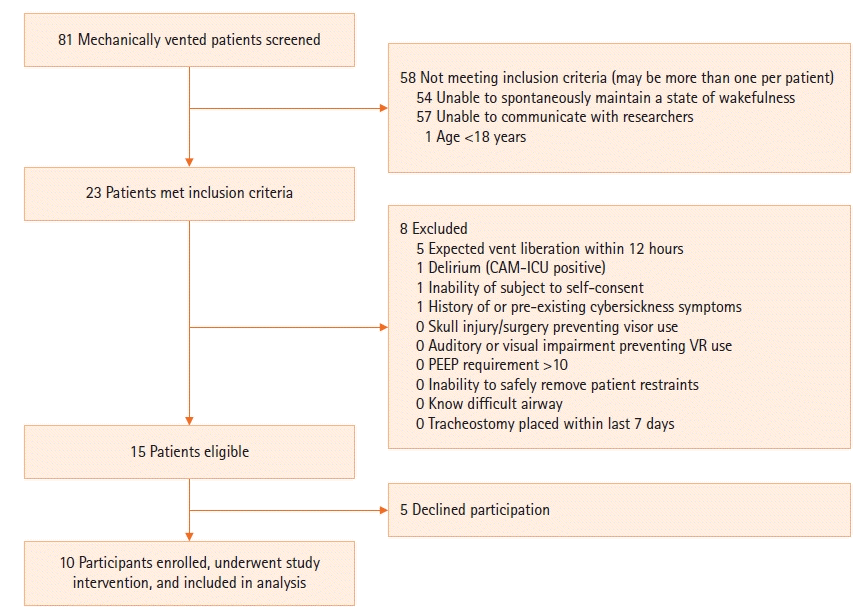
Table 1.
Primary and secondary outcome results
| Outcome | Value | P-valuea |
|---|---|---|
| Session during which a safety event occurred (n=18 sessions) | ||
| Cardiac arrhythmias (cardiac arrest or change to rhythm other than sinus or atrial fibrillation with rate <150) | 0 | |
| Hypotension (MAP <55) | 0 | |
| Hypertension (MAP >140) | 0 | |
| Oxygen desaturation (oxygen saturation < 85% for 3 minutes or greater) | 0 | |
| Fall from bed or chair | 0 | |
| Unintentional removal of medical device (lines, tubes, etc.) | 0 | |
| Session during which cybersickness occurred (n=18 sessions) | ||
| Subjective anxiety levels during session (100 point VAS-A scale) | ||
| Starting | 37 (19–74) | |
| Ending | 32 (7–64) | |
| Pairwise differenceb | –8.5 (–12.5 to –1.5) | 0.012 |
| Heart rate during session (beats/min) | ||
| Starting | 84 (71–88) | |
| Ending | 81 (72–79) | |
| Pairwise differenceb | 1 (–4 to 3) | 0.553 |
| Respiratory rate during session (beats/min) | ||
| Starting | 21 (18–26) | |
| Ending | 19 (17–24) | |
| Pairwise differenceb | –2 (–3 to 0) | 0.079 |
| Systolic blood pressure during session (mm Hg) | ||
| Starting | 130 (115–140) | |
| Ending | 118 (107–129) | |
| Pairwise differenceb | –9 (–17 to –1) | 0.003 |
| Diastolic blood pressure during session (mm Hg) | ||
| Starting | 70 (66–81) | |
| Ending | 67 (60–75) | |
| Pairwise differenceb | –4 (–12 to 1) | 0.040 |
| Reason for not performing planned sessions (n=5) | ||
| Subject preference or scheduling conflict with other therapies | 3 (60) | |
| Change in subject’s mental status | 1 (20) | |
| Symptom of cybersickness present prior to session start | 1 (20), dizziness |
Table 2.
Baseline characteristics of participants
| Variable | Value (n=10) |
|---|---|
| Age (yr) | 58 (49–66) |
| Birth sexa | |
| Female | 5 (50) |
| Male | 5 (50) |
| Racea | |
| African American or Black | 2 (20) |
| Caucasian | 8 (80) |
| Ethnicity | |
| Hispanic or Latino | 0 |
| Not Hispanic or Latino | 10 (100) |
| Reason for ICU admission | |
| Respiratory failure, ARDS | 0 |
| Respiratory failure, COPD exacerbation | 1 (10) |
| Respiratory failure, pneumonia | 4 (40) |
| Respiratory failure, pulmonary edema | 0 |
| Respiratory failure, CLAD | 2 (20) |
| Respiratory failure, other | 2 (20) |
| Shock, septic | 1 (10) |
| Shock, non-septic | 0 |
| Total duration of ICU stay (day) | 10.5 (6–14) |
| SOFA score at enrollmentb | 3 (2–3) |
| Airway | |
| Endotracheal tube | 6 (60) |
| Tracheostomy | 4 (40) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download