Abstract
Background
Morvan syndrome is characterized by neuromyotonia, dysautonomia, and various neuropsychiatric symptoms, as well as sleep disturbances, although these are less common than neuromuscular symptoms. Herein, we report a case of Morvan syndrome with peculiar sleep disturbances, documented via polysomnography.
Morvan syndrome is an autoimmune disorder characterized by the following: (1) central nervous system dysfunction, such as neuropsychiatric symptoms, insomnia, and myoclonus; (2) autonomic symptoms, such as diffuse sweating and arrhythmia; and (3) peripheral hyperactivity, such as myokymia or neuromyotonia [1]. Agrypnia excitata, which is characterized by a loss of sleep along with motor and autonomic hyperactivity, is linked to three conditions: Morvan syndrome, fatal familial insomnia, and delirium tremens [2]. As Morvan syndrome is extremely rare, polysomnographic findings of agrypnia excitata in Morvan syndrome are not well established. Herein, we report the case of a patient with Morvan syndrome who underwent 24-hour polysomnography (PSG) after presenting with confusion, agrypnia excitata, cramping, hyperhidrosis, and myokymia. The patient had previously undergone a thymectomy for the treatment of myasthenia gravis (MG).
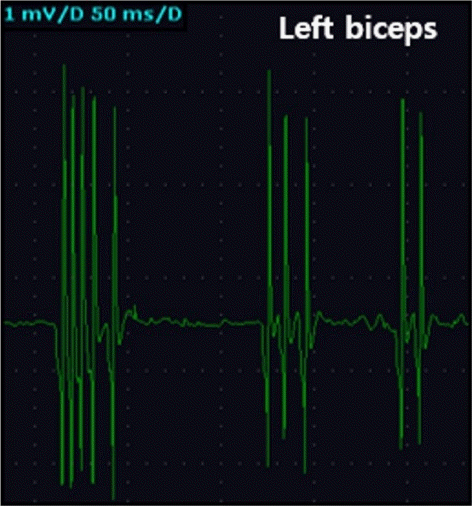
A 67-year-old man presented with a 4-month history of generalized weakness, diffuse sweating, muscle twitching, and tingling pain. The patient had developed fatigable ptosis and neck weakness 2 years prior. Due to a diagnosis of MG with thymoma, which was positive for anti-acetylcholine receptor antibodies, a thymectomy was performed for the management of uncontrolled symptoms, despite adequate treatment. Improvement in the ptosis and generalized weakness continued for four months post-thymectomy; however, this was followed by muscle twitching through the whole limb, pain in the right leg, and limb weakness. Upon his admission to the hospital, agitation, nighttime confusion, hyperhidrosis, and persistent diffuse limb myokymia were observed. Electromyography revealed fasciculation potentials and myokymic discharges in the biceps and gastrocnemius muscles (Fig. 1), although magnetic resonance imaging of his brain was unremarkable. Paraneoplastic antibodies (anti-Hu, Yo, Ri, amphiphysin, CV2/CRMP5, Ma2/Ta [PNMA2], recoverin, Sox1, and Titin) were found to be normal, but anti-voltage-gated potassium channel (VGKC)-complex antibodies, particularly the anti-CASPR2 and anti-LGI1 antibodies, were unavailable.
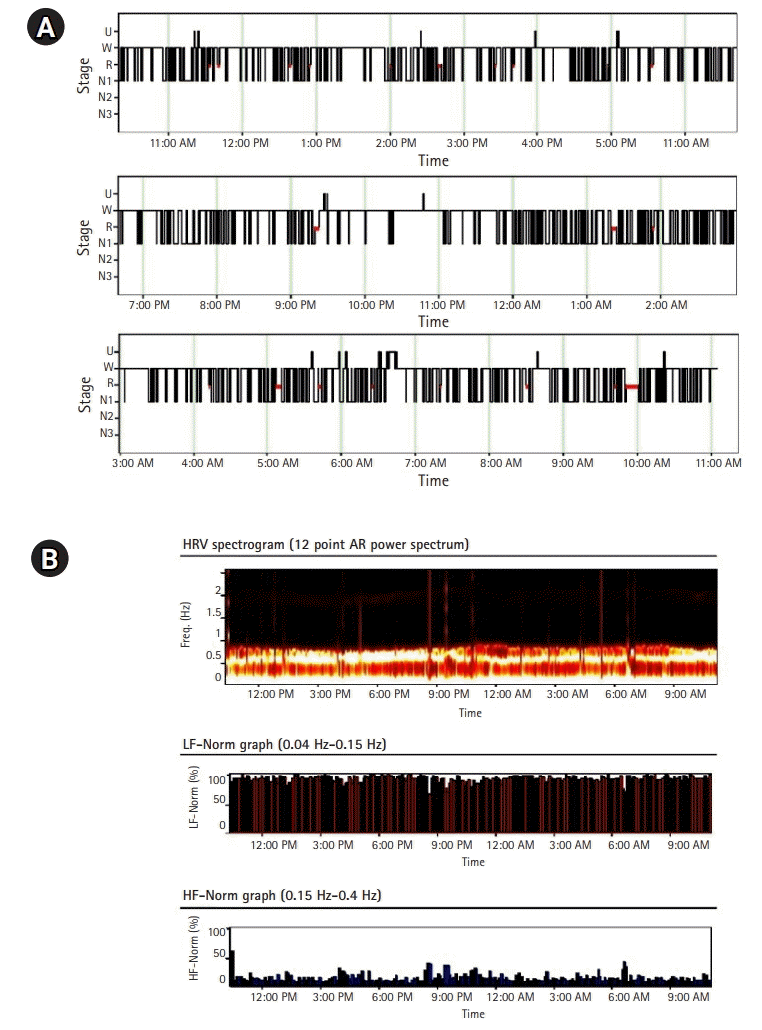
The patient complained of symptoms of severe insomnia, such as difficulty falling asleep and maintaining sleep, as well as repeated short bouts of falling sleep and waking up throughout the day. Moreover, the patient displayed nocturnal agitation and complex sleep behaviors, for which rapid eye movement (REM) sleep behavior disorder was a differential diagnosis. As the patient’s sleep-wake cycle seemed to be disrupted, the PSG study was performed over a 24-hour period, from 10:30 AM on the first day to 11:04 AM the next day (Fig. 2A). The patient slept for a total of 7 and 32 minutes over the 24-hour period. As sleep and wakefulness were irregularly distributed over the 24-hour period, a definite sleep-wake cycle could not be distinguished. The N1 and REM sleep durations were 405 minutes (89.6%) and 47 minutes (10.4%), respectively. Sleep spindles and K-complexes, which are N2 sleep and slow-wave sleep markers, respectively, were not observed. Complex movements of the hands and feet were consistently observed during both REM and non-REM sleep. Regarding REM-atonia dissociation, the absence of physiological REM-atonia was observed (tonic activity, 8.6%; phasic activity, 34.8%). Heart rate variability analysis revealed sympathetic hyperactivity (low-frequency power/high-frequency power, 10.25) (Fig. 2B). No sleep apnea was identified, nor were any movements which could be classified as periodic limb movement disorders. The characteristics of the patient’s PSG were consistent with those of agrypnia excitata (loss of sleep, confusion, and constant motor and autonomic hyperactivity). Treatment with gabapentin and carbamazepine improved neither the myokymia nor the tingling pain, and the patient was transferred to another hospital for palliative treatment.
Morvan syndrome, an autoimmune disorder characterized by central, autonomic, and peripheral hyperactivity, is found to be highly associated with thymoma [1]. Although the impact of thymectomy on the occurrence of autoimmune disease is variable, thymectomy and thymoma chemotherapy may trigger a disease, which suggests that thymic tumors may also harbor antigenic targets. In particular, CASPR2 [3] and anti-VGKC antibodies are thought to play a pathogenic role in peripheral and central nervous system symptoms. Recent studies have shown that tests specific to CASPR2 and LGI1 antibodies are becoming more important in Morvan syndrome than those specific to VGKC-complex antibodies [1,4]. It is necessary to accurately diagnose and determine a course of treatment through antibody testing CASPR2 and LGI1 in patients with thymoma- or post-thymectomy-associated peripheral nerve hyperexcitability syndrome [1,5]. Unfortunately, in the present case, serologic testing for CASPR2 and LGI1 antibodies was not performed, and intravenous immunoglobulin therapy was not administered.
Agrypnia excitata is thought to be due to the functional blocking of the thalamo-limbic circuits that regulate the sleep-wake cycle and bodily homeostasis by VGKC antibodies [1,5]. As the diencephalic and brainstem nuclei are involved in both arousal and autonomic homeostasis, disruption of this homeostasis can result in both insomnia and autonomic dysfunction. The results of a previous study supported the evidence that VGKC subfamilies are involved in sleep-wake cycle regulation [6], and indicated that mice lacking Kv-type potassium channels present with an increased motor drive and insomnia [7]. As in the case presented herein, agrypnia excitata is a key symptom differentiating Morvan syndrome from Isaac syndrome. Although a few studies largely support our findings, including recent polysomnographic studies in Korean men with fatal familial insomnia and a few studies with similar polysomnographic results [8-10], the results of present case suggest that widespread and small, fragmented blocks of sleep and wakefulness are not only mixed during the patient’s sleeping hours, but also continue over a 24-hour period.
In summary, we have presented the case of a male Korean patient with agrypnia excitata due to Morvan syndrome, diagnosed after a thymectomy for the treatment of MG. The features of agrypnia excitata found through PSG were (1) the disruption of a normal sleep-wake cycle throughout a 24-hour period, (2) the absence of sleep spindles and K-complexes, (3) the absence of slow-wave sleep, (4) complex actions during wakefulness and sleep, (5) increased muscle activity during REM and non-REM sleep, and (6) sympathetic hyperactivity. It is necessary to determine an accurate diagnosis and course of treatment by performing CASPR2 and LGI1 antibody tests in patients with thymoma- or post-thymectomy-associated peripheral nerve hyperexcitability syndrome and extended PSG testing.
Notes
ACKNOWLEDGMENTS
The present study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2020R1G1A1008446).
REFERENCES
1. Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012; 72:241–55.

3. Bernard C, Frih H, Pasquet F, Kerever S, Jamilloux Y, Tronc F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev. 2016; 15:82–92.

4. Michael S, Waters P, Irani SR. Stop testing for autoantibodies to the VGKC-complex: only request LGI1 and CASPR2. Pract Neurol. 2020; 20:377–84.

5. Montagna P, Lugaresi E. Agrypnia Excitata: a generalized overactivity syndrome and a useful concept in the neurophysiopathology of sleep. Clin Neurophysiol. 2002; 113:552–60.

6. Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, et al. Reduced sleep in drosophila shaker mutants. Nature. 2005; 434:1087–92.

7. Espinosa F, Marks G, Heintz N, Joho RH. Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels. Genes Brain Behav. 2004; 3:90–100.

8. Abgrall G, Demeret S, Rohaut B, Leu-Semenescu S, Arnulf I. Status dissociates and disturbed dreaming in a patient with Morvan syndrome plus myasthenia gravis. Sleep Med. 2015; 16:894–6.
9. Cornelius JR, Pittock SJ, McKeon A, Lennon VA, Aston PA, Josephs KA, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011; 68:733–8.

10. Yang TW, Park B, Kim KT, Jun JS, Kim YS, Lee ST, et al. Fatal familial insomnia presenting with agrypnia excitata and very low atonia index level: a case report and literature review. Medicine (Baltimore). 2018; 97:e0646.
Fig. 2.
(A) Hypnography over a 24-hour period and (B) heart rate variability (HRV) analysis. Widespread and small, fragmented block of sleep throughout a 24-hour period were identified. Note rapid eye movement sleep occupying most of the sleep period. U, undetermined sleep stage; W, wakefulness; R, rapid eye movement sleep; N, non-rapid eye movement sleep; AR, autoregressive; Freq, frequency; LF-Norm, normalized low frequency; HF-Norm, normalized high frequency.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download