Abstract
Background
Ischemic stroke is one of the serious neurological complications of coronavirus disease 2019 (COVID-19). However, ischemic stroke can develop secondary complications after cardiac involvement in COVID-19.
Case Report
We report the case of a 22-year-old patient who presented with malignant cerebral infarction 10 months after COVID-19-related myocarditis. A 22-year-old woman was referred to the emergency room because of abnormal mental status changes. She developed heart failure and arrhythmia after COVID-19-related myocarditis. Brain magnetic resonance imaging (MRI) revealed high signal intensity on diffusion-weighted imaging that was indicative of acute cerebral infarction in the left middle cerebral artery (MCA) and left anterior cerebral artery (ACA) territory. In addition, occlusion of both the left MCA and ACA was observed on brain MRI. Craniectomy with therapeutic hypothermia was performed to treat the cerebral edema.
Since the first case of pneumonia caused by coronavirus disease 2019 (COVID-19) was detected in Wuhan City in China in December 2019, COVID-19 has been the most prevalent infectious disease in the modern medicine era [1]. Although COVID-19 mainly causes respiratory infection, another wide spectrum of clinical diseases, including cardiovascular and neurological complications that the virus itself causes, have been elucidated [2,3]. In particular, ischemic stroke is one of the neurological complications of COVID-19. It could be caused by coagulation abnormalities or vascular endothelial injury caused by the virus [4]. However, cardioembolic stroke, which accounts for approximately 20% of the total causes of ischemic stroke, could develop secondary manifestations of cardiac involvement of the virus [5]. Herein, we report the case of a 22-year-old patient who presented with malignant cerebral infarction 10 months after COVID-19-related myocarditis.
A 22-year-old woman was referred to our institution’s emergency room because of a sudden change in mental status. She had a history of COVID-19, which she had contracted during a COVID-19 outbreak in Daegu, South Korea, 10 months earlier. On the patient’s initial admission for COVID-19, the main complaint was acute chest pain with dyspnea. On physical examination, her blood pressure was 100/60 mmHg, and body temperature was 38°C. Considering that the entire world is amid the COVID-19 pandemic, a polymerase chain reaction test for COVID-19 using a nasopharyngeal swab was performed, and the result came back positive. Laboratory examination revealed a lymphocyte-dominant white blood cell count of 1,212 uL. The C-reactive protein level was 0.6 mg/dL. In addition, brain natriuretic peptide and troponin I levels were 1,929 pg/mL (reference range, <125 pg/mL) and 1.26 ng/mL (reference range, <0.3 ng/mL), respectively. Chest radiography revealed diffuse multifocal consolidation in both lung fields with cardiomegaly. Electrocardiography (EKG) revealed an intraventricular conduction delay and premature complexes. Echocardiography revealed severe left ventricular dysfunction.
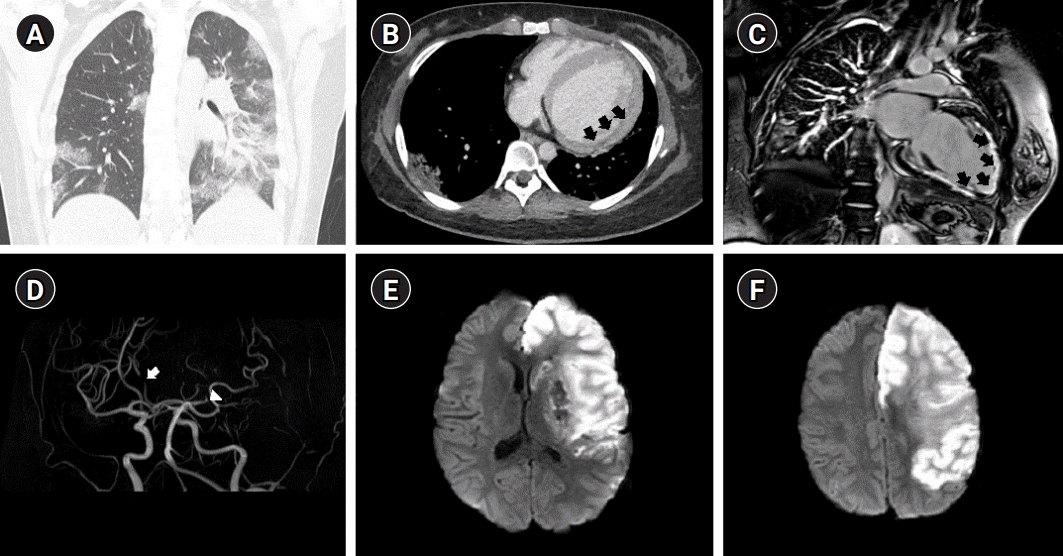
Multimodal imaging was performed to diagnose COVID-19-related myocarditis. A biopsy is a helpful and precise method for diagnosing myocarditis [6]; however, it cannot be performed because of the risk of COVID-19 infection. Cardiac computed tomography (CT) showed multifocal consolidation with ground-glass opacities in the lungs (Fig. 1A) and hypertrophied myocardium due to edema (Fig. 1B). Cardiac magnetic resonance imaging (MRI) showed transmural late gadolinium enhancement (Fig. 1C), indicative of myocarditis. Treatment was initiated with lopinavir/ritonavir for seven days with diuretics. At the time of discharge, the ejection fraction was 28%, with severely decreased global wall motion in the left ventricle (LV) and a dilated left atrium (LA) noted on transthoracic echocardiography (TTE; LA volume index, 58.3; LV volume index, 107.78). Nonsustained ventricular tachycardia was also observed. Subsequently, she was followed up in the outpatient department and treated with sacubitril/valsartan and diuretics. During follow-up, EKGs were performed frequently, no atrial fibrillation (AF) was detected, no intracardiac thrombus was observed, and no significant change in the LA and LV volume index on TTE was noted.
On her second most recent admission for cerebral infarction, the patient saw her parents off to work at 8:00 AM. Her parents discovered her 10 hours later with an altered mental status. On neurological examination, the patient was drowsy with notable global aphasia. According to the Medical Research Council grading system, the power of the right upper extremity was 1/5, whereas that of the right lower extremity was 2/5. The total National Institute of Health Stroke Scale score was 18 (level of consciousness [LOC] question, 2; LOC command, 2; best gaze, 2; facial palsy, 2; right arm motor, 2; right leg motor, 2; sensory, 1; best language, 3; dysarthria, 2). EKG revealed AF that was not previously detected during the follow-up examinations in the outpatient department. Diffusion-weighted brain MR revealed high signal intensity in the left middle cerebral artery (MCA) and left anterior cerebral artery (ACA) (Fig. 1D). MR angiography demonstrated severe stenosis of the left ACA and occlusion of the left MCA (Fig. 1E). One day after admission, a follow-up brain CT scan indicated that a subfalcine herniation had developed (Fig. 1F), and craniectomy was immediately performed.
To manage brain edema after craniectomy, intravenous D-mannitol was administered, and therapeutic hypothermia was applied using a surface cooling device (Arctic Sun) at a target temperature of 34°C. Light sedation with dexmedetomidine was administered during hypothermic induction, and the target mean arterial pressure was set at >60 mmHg to maintain cerebral perfusion pressure. Acetaminophen and buspirone were administered to control the shivering. If the two drugs were unable to control the shivering, additional intravenous pethidine was administered. The heart rhythm was controlled using intravenous amiodarone (class III, anti-arrhythmic drug), and furosemide was administered to prevent fluid retention. Gradually, her mental status improved from drowsiness to alertness when she was moved from the intensive care unit to the general ward. On TTE, the LA volume index increased to 80.41 Compared with previous findings (previous LA volume index, 58.3). The patient was discharged on apixaban for anticoagulation, sacubitril/valsartan, diuretics, and amiodarone one month after admission. Three months after discharge, the patient was able to stand with assistance, and her global aphasia improved to transcortical motor aphasia. Despite neurological improvements, there was no improvement in cardiac function based on TTE findings compared to the findings at discharge.
COVID-19 can directly invade the myocardium and cause myocarditis [7]. This may be due to the affinity of the virus for angiotensin-converting enzyme 2 (ACE2), which acts as a portal for viral entry and ACE2 downregulation, leading to myocardial dysfunction [8]. Therefore, myocarditis should be suspected in patients with COVID-19 with acute chest pain and elevated cardiac enzyme levels. In addition, myocarditis is associated with secondary complications such as malignant tachycardia and heart failure [9]. In our case, the patient exhibited acute chest pain and elevated cardiac enzyme levels after COVID-19 infection; myocarditis was confirmed by cardiac CT and MRI [10]. At discharge, the patient had severely decreased LV wall motion, and a dilated LA was noted on TTE with recurrent ventricular tachycardia. Additionally, AF was noted in the patients in the emergency room. All of these conditions are high-risk cardioembolic causes of ischemic stroke. Therefore, fulminant complications of COVID-19-related myocarditis could have contributed to the development of ischemic stroke in our patient.
In this case, AF was not documented during frequent EKG monitoring in the outpatient department. However, the patient also had several high-risk cardioembolic sources. Therefore, preventive anticoagulation therapy should be considered in such cases. In this regard, there have been several reports on the benefits of preventive anticoagulation for heart failure [11]. In addition, to find the cover AF, frequent 24-hour Holter monitoring or an implantable loop recorder should be considered.
The cumulative number of confirmed COVID-19 cases worldwide has surpassed four billion. This means that the number of survivors with secondary complications after COVID-19 is expected to increase proportionally. Therefore, preventing disease transmission is imperative; however, focusing on survivors with secondary complications after COVID-19 may also be crucial because the long-term sequelae of COVID-19 may lead to serious conditions such as ischemic stroke.
Further analysis is vital to clarify the extent of COVID-19-related cardiovascular complications and prevent the occurrence of ischemic stroke. In addition, prophylactic anticoagulation should be considered in patients at a high risk of venous and arterial thromboembolism after COVID-19 infection.
Notes
Ethics statement
This study was reviewed and approved by the Institutional Review Board of Keimyung University Dongsan Hospital (IRB No. 2002-03-007). The requirement for informed consent was waived.
Conflict of interest
Jeong-Ho Hong is an editorial board member of the journal but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
1. World Health Organization. Pneumonia of unknown cause: China. 2020. Geneva: World Health Organization;2021. [cited 2022 May 1]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229.
2. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020; 5:831–40.

3. Sharifian-Dorche M, Huot P, Osherov M, Wen D, Saveriano A, Giacomini PS, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020; 417:117085.

4. Montalvan V, Toledo J, Nugent K. Mechanisms of stroke in coronavirus disease 2019. J Stroke. 2020; 22:282–3.

5. Kim JY, Kang K, Kang J, Koo J, Kim DH, Kim BJ, et al. Executive summary of stroke statistics in Korea 2018: a report from the epidemiology research council of the Korean Stroke Society. J Stroke. 2019; 21:42–59.

6. Caforio AL, Baritussio A, Basso C, Marcolongo R. Clinically suspected and biopsy-proven myocarditis temporally associated with SARS-CoV-2 infection. Annu Rev Med. 2022; 73:149–66.

8. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020; 383:120–8.

9. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020; 75:2352–71.

Fig. 1.
(A) Chest computed tomography showing multifocal consolidation with ground-glass opacities in both lungs. (B) Cardiac magnetic resonance imaging showing a hypertrophied myocardium due to edema (black arrows). (C) Cardiac magnetic resonance imaging showing transmural late gadolinium enhancement (black arrows). (D) Brain magnetic resonance angiography showing the severe stenosis of the left A2 segment of the anterior cerebral artery (ACA; white arrow) and the distal segment of the left middle cerebral artery (MCA; white arrowhead). (E, F) A diffusion-weighted image showing high signal intensity in the left MCA and ACA territory infarction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download