1. Hamashima C, Ogoshi K, Narisawa R, et al. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol. 2015; 21:2460–2466.
2. Cohen J, Safdi MA, Deal SE, et al. Quality indicators for esophagogastroduodenoscopy. Am J Gastroenterol. 2006; 101:886–891.
3. Park WG, Shaheen NJ, Cohen J, et al. Quality indicators for EGD. Gastrointest Endosc. 2015; 81:17–30.
4. Park CH, Kim B, Chung H, et al. Endoscopic quality indicators for esophagogastroduodenoscopy in gastric cancer screening. Dig Dis Sci. 2015; 60:38–46.
5. Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. 2017; 66:1886–1899.
6. Min JK, Cha JM, Cho YK, et al. Revision of quality indicators for the endoscopy quality improvement program of the National Cancer Screening Program in Korea. Clin Endosc. 2018; 51:239–252.
7. Chiu PWY, Uedo N, Singh R, et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. 2019; 68:186–197.
8. Bisschops R, Areia M, Coron E, et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016; 48:843–864.
9. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010; 362:1795–1803.
10. Keswani RN, Crockett SD, Calderwood AH. AGA clinical practice update on strategies to improve quality of screening and surveillance colonoscopy: expert review. Gastroenterology. 2021; 161:701–711.
11. Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006; 355:2533–2541.
12. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015; 81:31–53.
13. Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J Clin Pathol. 2002; 55:770–773.
14. East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut. 2015; 64:991–1000.
15. Ren W, Yu J, Zhang ZM, et al. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol. 2013; 19:2092–2096.
16. Chadwick G, Groene O, Hoare J, et al. A population-based, retrospective, cohort study of esophageal cancer missed at endoscopy. Endoscopy. 2014; 46:553–560.
17. Pimenta-Melo AR, Monteiro-Soares M, Libânio D, et al. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016; 28:1041–1049.
18. Beck M, Bringeland EA, Qvigstad G, et al. Gastric cancers missed at upper endoscopy in Central Norway 2007 to 2016: a population-based study. Cancers (Basel). 2021; 13:5628.
19. Uedo N, Gotoda T, Yoshinaga S, et al. Differences in routine esophagogastroduodenoscopy between Japanese and international facilities: a questionnaire survey. Dig Endosc. 2016; 28 Suppl 1:16–24.
20. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003; 58:76–79.
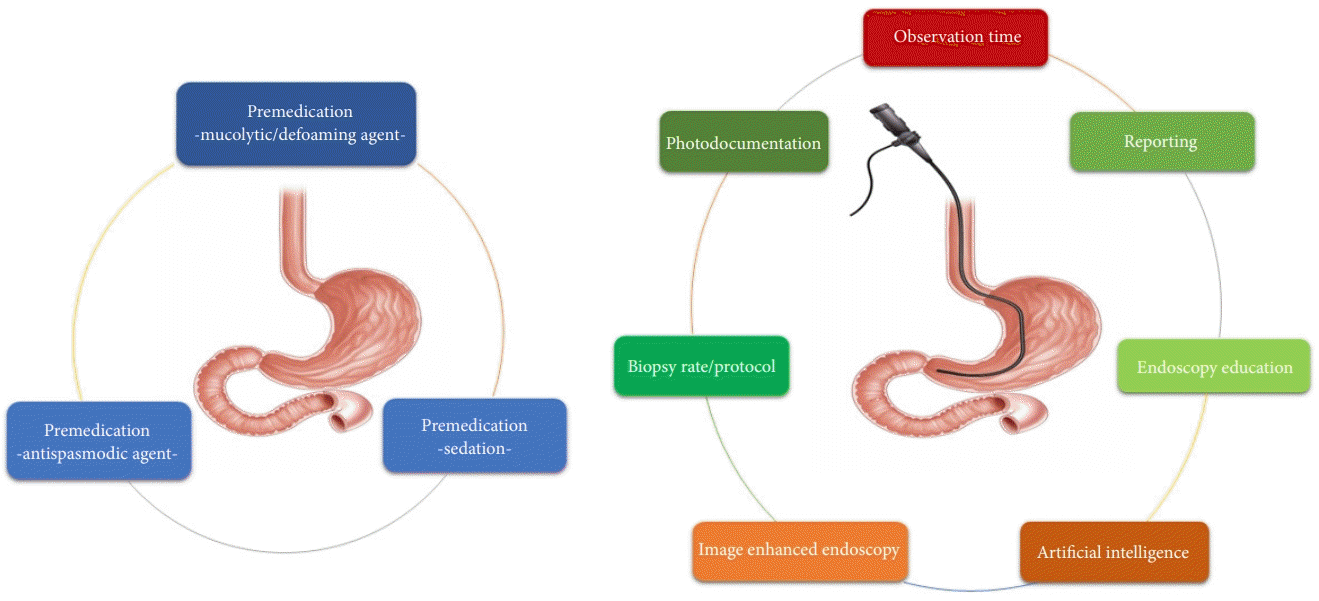
21. Neale JR, James S, Callaghan J, et al. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: a randomized, controlled, endoscopist-blinded study. Eur J Gastroenterol Hepatol. 2013; 25:778–783.
22. Lee GJ, Park SJ, Kim SJ, et al. Effectiveness of premedication with pronase for visualization of the mucosa during endoscopy: a randomized, controlled trial. Clin Endosc. 2012; 45:161–164.
23. Liu X, Guan CT, Xue LY, et al. Effect of premedication on lesion detection rate and visualization of the mucosa during upper gastrointestinal endoscopy: a multicenter large sample randomized controlled double-blind study. Surg Endosc. 2018; 32:3548–3556.
24. Chang WK, Yeh MK, Hsu HC, et al. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol. 2014; 29:769–774.
25. Zhang Q, Chen ZY, Chen CD, et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: an observational study in China. Medicine (Baltimore). 2015; 94:e384.
26. Kim SY, Park JM, Cho HS, et al. Assessment of cimetropium bromide use for the detection of gastric neoplasms during esophagogastroduodenoscopy. JAMA Netw Open. 2022; 5:e223827.
27. Omata F, Kumakura Y, Ishii N, et al. Noneffectiveness of scopolamine for facilitating detection of upper gastrointestinal neoplasia during screening esophagogastroduodenoscopy: propensity score-matched study. Endoscopy. 2020; 52:556–562.
28. Cohen LB, Wecsler JS, Gaetano JN, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006; 101:967–974.
29. McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008; 67:910–923.
30. Bell GD, Quine A. Preparation, premedication, and surveillance. Endoscopy. 2006; 38:105–109.
31. Fanti L, Testoni PA. Sedation and analgesia in gastrointestinal endoscopy: what’s new? World J Gastroenterol. 2010; 16:2451–2457.
32. Zhou J, Li Z, Ji R, et al. Influence of sedation on the detection rate of early cancer and precancerous lesions during diagnostic upper gastrointestinal endoscopies: a multicenter retrospective study. Am J Gastroenterol. 2021; 116:1230–1237.
33. Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013; 26:11–22.
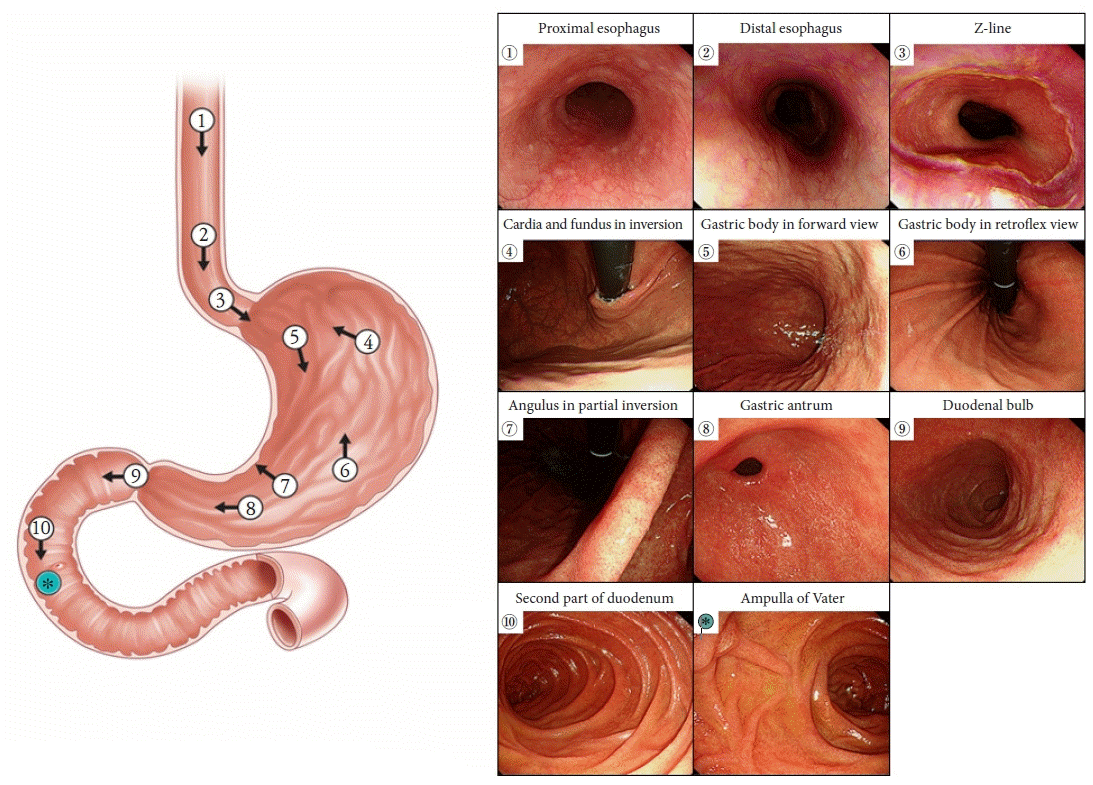
34. Lee JI, Kim JS, Kim BW, et al. Taking more gastroscopy images increases the detection rate of clinically significant gastric lesions: validation of a systematic screening protocol for the stomach. Korean J Helicobacter Up Gastrointest Res. 2020; 20:225–232.
35. Park JM, Lim CH, Cho YK, et al. The effect of photo-documentation of the ampulla on neoplasm detection rate during esophagogastroduodenoscopy. Endoscopy. 2019; 51:115–124.
36. Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996; 111:85–92.
37. Alvarez Herrero L, Curvers WL, van Vilsteren FG, et al. Validation of the Prague C&M classification of Barrett’s esophagus in clinical practice. Endoscopy. 2013; 45:876–882.
38. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974; 2:394–397.
39. Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005; 37:570–578.
40. Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013; 62:489–495.
41. Cheng HT, Cheng CL, Lin CH, et al. Caustic ingestion in adults: the role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008; 8:31.
42. de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63:743–752.
43. Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008; 22:601–616.
44. Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989; 2:783–785.
45. Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus. Gastrointest Endosc. 2012; 76:531–538.
46. Teh JL, Tan JR, Lau LJ, et al. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol. 2015; 13:480–487.
47. Kawamura T, Wada H, Sakiyama N, et al. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig Endosc. 2017; 29:569–575.
48. Park JM, Huo SM, Lee HH, et al. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017; 153:460–469.
49. Park JM, Kim SY, Shin GY, et al. Implementation effect of institutional policy of EGD observation time on neoplasm detection. Gastrointest Endosc. 2021; 93:1152–1159.
50. Lee HH, Park JM, Lim CH, et al. The impact of pre-resection endoscopic examination time on the rate of synchronous gastric neoplasms missed during endoscopic treatment. Surg Endosc. 2017; 31:3952–3960.
51. Choi Y, Choi HS, Jeon WK, et al. Optimal number of endoscopic biopsies in diagnosis of advanced gastric and colorectal cancer. J Korean Med Sci. 2012; 27:36–39.
52. Hatfield AR, Slavin G, Segal AW, et al. Importance of the site of endoscopic gastric biopsy in ulcerating lesions of the stomach. Gut. 1975; 16:884–886.
53. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019; 51:365–388.
54. Januszewicz W, Wieszczy P, Bialek A, et al. Endoscopist biopsy rate as a quality indicator for outpatient gastroscopy: a multicenter cohort study with validation. Gastrointest Endosc. 2019; 89:1141–1149.
55. Lee HS, Jeon SW. Barrett esophagus in Asia: same disease with different pattern. Clin Endosc. 2014; 47:15–22.
56. Rex DK, Shaw M, Wong R. Prevalence of Barrett’s esophagus. Gastroenterology. 2006; 130:1373–1374.
57. Levine DS. Management of dysplasia in the columnar-lined esophagus. Gastroenterol Clin North Am. 1997; 26:613–634.
58. Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–1181.
59. Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018; 21:579–587.
60. Yamazato T, Oyama T, Yoshida T, et al. Two years’ intensive training in endoscopic diagnosis facilitates detection of early gastric cancer. Intern Med. 2012; 51:1461–1465.
61. Korean Society of Gastrointestinal Endoscopy. Quality Control E-learning [Internet]. Seoul: The Korean Society of Gastrointestinal Endoscopy;2020. [cited 2022 Mar 9]. Available from:
https://www.gie.or.kr/disinfect/Elearning.php.
62. National Cancer Center. National Cancer Screening Quality Improvement Online Education Program [Internet]. Goyang: National Cancer Center;2013. [cited 2022 Mar 9]. Available from:
https://neweducation.ncc.re.kr/#.
63. Lee W. Application of current image-enhanced endoscopy in gastric diseases. Clin Endosc. 2021; 54:477–487.
64. Mabe K, Yao K, Nojima M, et al. An educational intervention to improve the endoscopist’s ability to correctly diagnose small gastric lesions using magnifying endoscopy with narrow-band imaging. Ann Gastroenterol. 2014; 27:149–155.
65. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019; 25:1666–1683.
66. Wu L, He X, Liu M, et al. Evaluation of the effects of an artificial intelligence system on endoscopy quality and preliminary testing of its performance in detecting early gastric cancer: a randomized controlled trial. Endoscopy. 2021; 53:1199–1207.
67. Kuo CY, Chiu HM. Application of artificial intelligence in gastroenterology: potential role in clinical practice. J Gastroenterol Hepatol. 2021; 36:267–272.
68. Bang CS, Lee JJ, Baik GH. Computer-aided diagnosis of esophageal cancer and neoplasms in endoscopic images: a systematic review and meta-analysis of diagnostic test accuracy. Gastrointest Endosc. 2021; 93:1006–1015.
69. Choi SJ, Khan MA, Choi HS, et al. Development of artificial intelligence system for quality control of photo documentation in esophagogastroduodenoscopy. Surg Endosc. 2022; 36:57–65.
70. Wu L, Zhang J, Zhou W, et al. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut. 2019; 68:2161–2169.
71. Hirasawa T, Aoyama K, Tanimoto T, et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018; 21:653–660.
72. Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? a meta-analysis. Endosc Int Open. 2014; 2:E46–E50.
73. Yalamarthi S, Witherspoon P, McCole D, et al. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004; 36:874–879.
74. Chen D, Wu L, Li Y, et al. Comparing blind spots of unsedated ultrafine, sedated, and unsedated conventional gastroscopy with and without artificial intelligence: a prospective, single-blind, 3-parallel-group, randomized, single-center trial. Gastrointest Endosc. 2020; 91:332–339.
75. Berzin TM, Parasa S, Wallace MB, et al. Position statement on priorities for artificial intelligence in GI endoscopy: a report by the ASGE Task Force. Gastrointest Endosc. 2020; 92:951–959.
76. Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017; 20:217–225.
77. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009; 58:331–336.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download