Abstract
Background/Aims
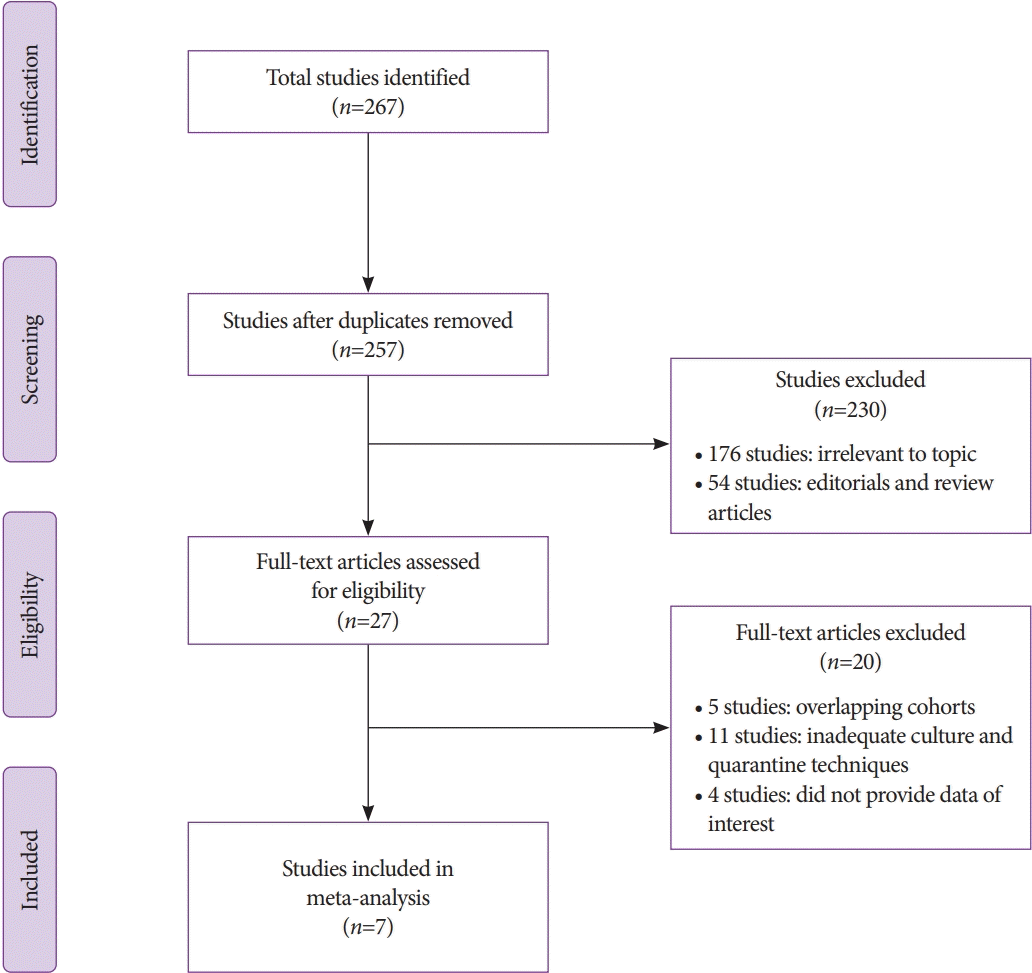
Methods
Results
Notes
Conflicts of Interest: Richard A Kozarek is a Research support for Boston Scientific and NIH. Andrew Ross is a consultant for Boston Scientific. The other authors have no potential conflicts of interest.
Author Contributions
Conceptualization : Shivanand Bomman, Rajesh Krishnamoorthi
Data curation: SB, Navroop Nagra, Shruti Chandra
Formal analysis: SB, Munish Ashat, NN, Mahendran Jayaraj, RK
Investigation: SB, NN, MJ, SC
Methodology: SB, RK
Supervision: Richard A Kozarek, Andrew Ross
Validation: SB, MA, NN, MJ, SC, RAK, AR, RK
Visualization: SB, NN
Writing-original draft: SB, RK
Writing-review&editing: SB, MA, NN, MJ, SC, RAK, AR, RK
REFERENCES
Fig. 2.
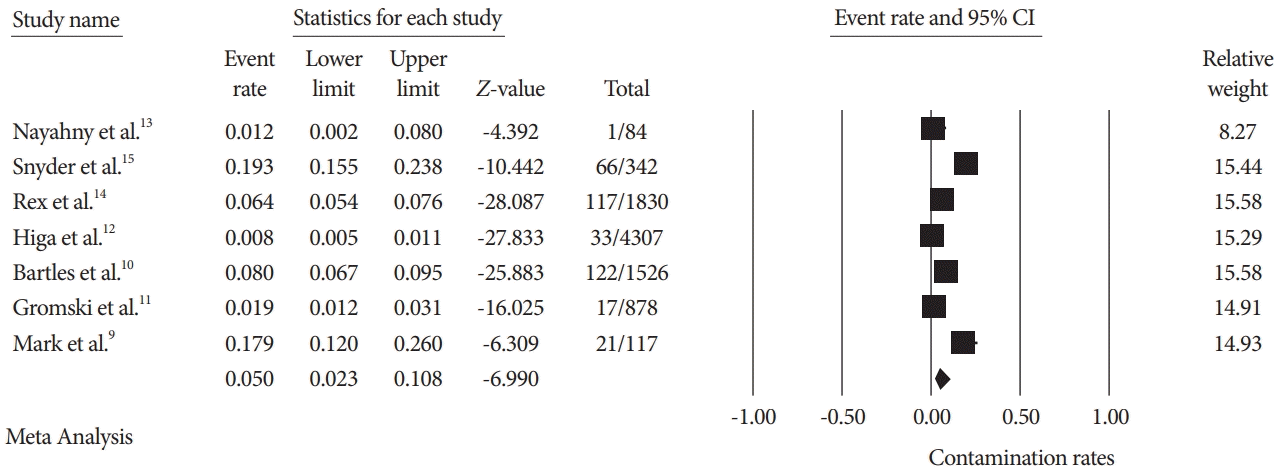
Fig. 3.
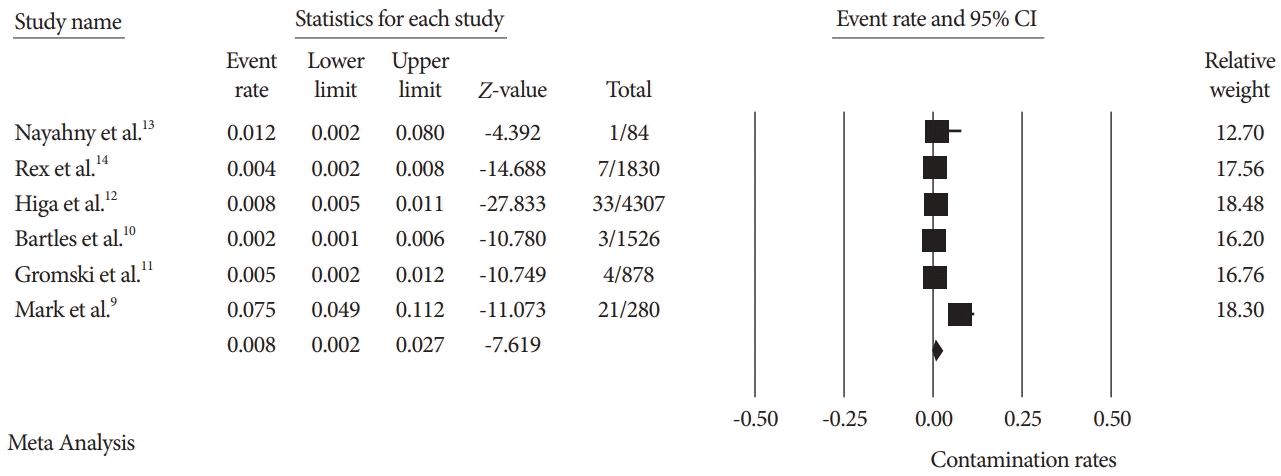
Fig. 4.
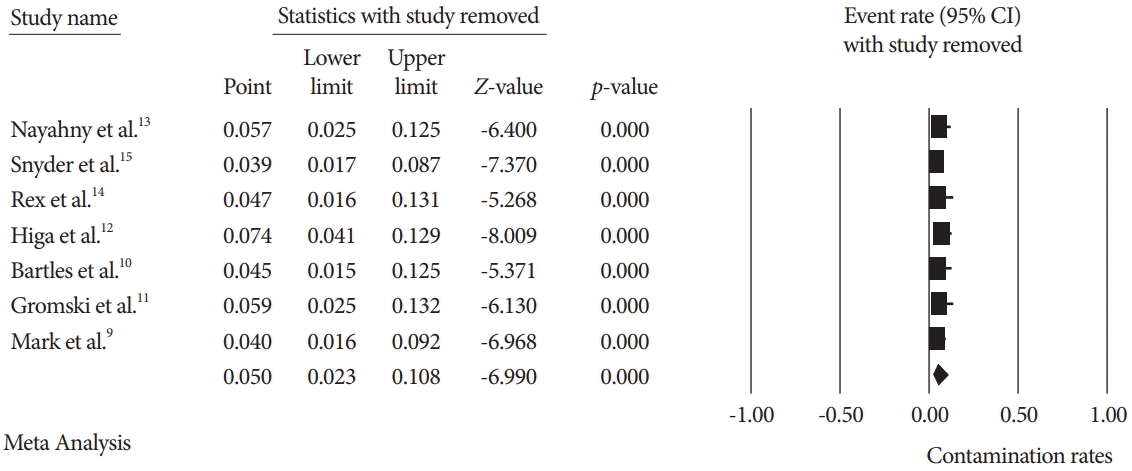
Table 1.
| Authors (year) | Study type | Positive cultures (%) | Number of scopes cultured (n) | Reprocessing technique used | High-risk organism % |
|---|---|---|---|---|---|
| Naryzhny et al. (2016) [13] | Single site, prospective study | 1 (1.19%) | 84 | EtO | 1.19% |
| Snyder et al. (2017) [15] | Single site, prospective randomized study | 66 (19.29%) | 342 | EtO and dHLD | N/A |
| Rex et al. (2017) [14] | Single site, prospective study | 117 (6.87%) | 1830 | dHLD | 0.49% |
| Higa et al. (2018) [12] | Single site, retrospective study | 33 (0.69%) | 4307 | Culture and quarantine | 0.69% |
| Bartles et al. (2018) [10] | Multisite, prospective randomized study | 122 (7.99%) | 1526 | dHLD | 0.19% |
| Gromski et al. (2020) [11] | Single site, prospective randomized study | 17 (1.93%) | 878 | dHLD and liquid sterilant | 0.45% |
| Mark et al. (2020) [9] | Single site, prospective study | 21 (18%) | 117 | Culture and quarantine | 7.5% |
High-risk organisms are more often associated with disease, such as Gram-negative bacteria (e.g., Escherichia coli, Klebsiella pneumoniae, or other Enterobacteriaceae, as well as Pseudomonas aeruginosa), Staphylococcus aureus, and Enterococcus.
dHLD, double high-level disinfection; EtO, ethylene oxide sterilization; N/A, not available.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download