Abstract
Background/Aims
Endoscopic band ligation (EBL) is used to treat colonic diverticular bleeding (CDB). An endoscopic variceal ligation device for esophageal varices is used as a conventional EBL device (C-EBL). A new EBL device (N-EBL) was developed by Sumitomo Bakelite Co. in August 2018. We aimed to evaluate the clinical outcomes of N-EBL compared with those of C-EBL.
Methods
Seventy-nine patients who underwent EBL for CDB at St. Luke’s International Hospital, Japan, between 2017 and 2020 were included in this retrospective study. Patients were divided into the C-EBL and N-EBL groups. Their clinical outcomes, including achieving initial hemostasis, early rebleeding, procedure time, and EBL-associated adverse events, were evaluated.
Results
Of the 79 patients, 36 (45.6%) were in the C-EBL group and 43 (54.4%) were in the N-EBL group. The rate of achieving initial hemostasis was 100% in the C-EBL group and 93.0% in the N-EBL group. No significant difference was noted in the early rebleeding rate between the groups (p=0.24). The N-EBL group achieved a shorter median EBL procedure time than the C-EBL group (14.2 minutes vs. 18.2 minutes, p=0.02). No adverse events were observed in either group.
Colnic diverticular bleeding (CDB) is the most frequent cause of lower gastrointestinal bleeding.1 Several endoscopic techniques, including epinephrine injection, thermal coagulation, endoscopic clipping (EC), endoscopic band ligation (EBL), and over-the-scope clip, have been used for the treatment of CDB. The EBL method has been reported to be beneficial in treating CDB, with a lower rebleeding rate than conventional treatment, including epinephrine injection, thermal coagulation, and EC, and elimination of the diverticulum after EBL.2-4 The EBL method was described as a treatment for CDB in the guidelines for CDB and colonic diverticulitis published by the Japan Gastroenterological Association in 2018.5 Until now, the esophageal endoscopic variceal ligation (EVL) device (MD48710 U; Sumitomo Bakelite Co., Tokyo, Japan) has been used for CDB as a conventional EBL device (C-EBL). However, in August 2018, a new EBL device (N-EBL) for CDB was developed by the company with regulatory approval. The N-EBL device has the following characteristics: it includes three types of devices according to the endoscope diameter, is approved for the treatment of not only CDB but also internal hemorrhoids, and provides a wider field of vision than C-EBL. The N-EBL device has been widely used for the treatment of CDB; however, detailed information and comparative studies have not been reported.
Therefore, this retrospective cohort study aimed to compare the clinical outcomes between C-EBL and N-EBL for the treatment of CDB.
We conducted a retrospective study at St. Luke’s International Hospital, a tertiary referral center in Tokyo, Japan, between April 2017 and February 2020. A total of 79 patients who were treated with EBL as the first-line treatment for definitive CDB were included.
We divided the patients into two groups before and after the introduction of the new device (August 2018): the C-EBL group was treated with the conventional EBL device and the N-EBL group was treated with the new EBL device. The primary outcome was the rate of early rebleeding within 30 days after EBL. The secondary outcomes were the rate of initial achieving hemostasis, the rate of complete inversion of the diverticulum after EBL, procedure time, and adverse events such as perforation and diverticulitis.
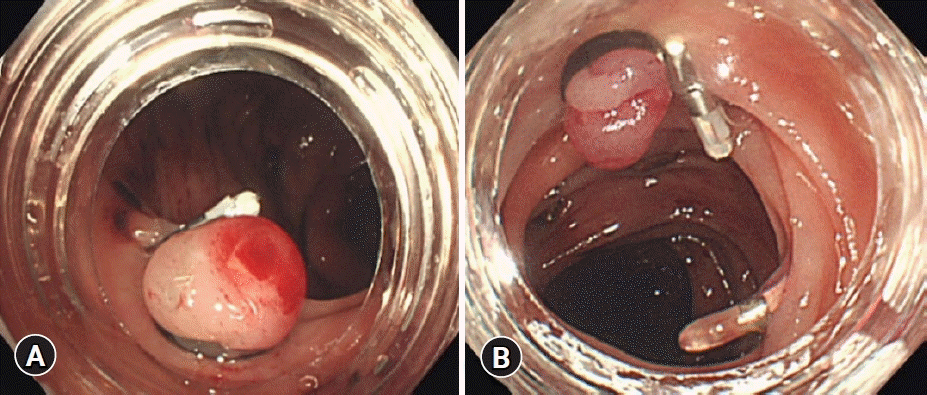
The characteristics of both devices used in this study are shown in Table 1 and Figure 1. The N-EBL, developed by Sumitomo Bakelite Co., is available in three types, depending on the outer diameter of the adaptive endoscope, making it highly versatile for different types of colonoscopes: MD-48910B (10.5–12.0 mm), MD-48912B (12.2–13.0 mm), and MD-48913B (13.1–13.8 mm). We used the MD-48912B N-EBL in this study in accordance with the endoscopes adopted in our hospital. The device is approved for the treatment of CDB and internal hemorrhoids. Moreover, this device provides a wider field vision than C-EBL (Fig. 2).
Colonoscopy was performed using a water-jet scope (PCF-Q260AZI, PCF-Q260JI, PCF-H290I, or GIF-Q260J; Olympus Medical Systems, Tokyo, Japan). A transparent hood (D201-12704; Olympus Medical Systems) was attached to the endoscope. Bowel preparation was performed in all cases except when vital signs were unstable. When a diverticulum with stigmata of recent hemorrhage (SRH) was identified, hemoclips (HX-610-135; Olympus Medical Systems) were applied as markers near the responsible diverticulum, and the endoscope was removed. The endoscope was re-inserted after attaching a band ligator device (MD-48710 EVL device in the C-EBL group and MD48912B EBL device in the N-EBL group). The responsible diverticulum was suctioned into the hood of the band ligator device, and an elastic O-ring was deployed.6-9 Colonoscopy was performed by expert or non-expert endoscopists under supervision. Expert endoscopists were defined as the institutional teaching staff of St. Luke’s International Hospital who were also board-certified members of the Japanese Society of Gastroenterology and had performed more than 500 routine colonoscopies. None of the nonexpert endoscopists were board-certified members of the Japanese Society of Gastroenterology; however, they had performed >500 routine colonoscopies before performing endoscopic hemostasis. Patients were followed up at our outpatient clinic for at least 30 days after EBL.
The following data were obtained from patients’ medical records: age, sex, blood transfusion, location of hemorrhage, the type of SRH, inversion of the diverticula after EBL, extravasation on computed tomography, and EBL-associated adverse events.
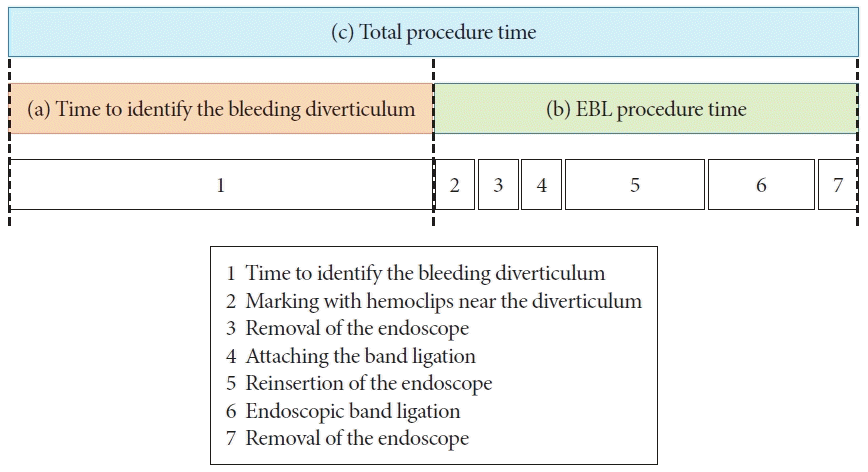
Definite CDB was defined as CDB with SRH. SRH was defined as the visualization of active bleeding, a non-bleeding visible vessel, and an adherent clot.10 We defined rebleeding as the presence of a significant quantity of fresh blood loss or passage of wine-colored stools after colonoscopy. Complete inversion was defined as the presence of the apex of the diverticulum in the intestinal lumen after EBL, and incomplete inversion was defined as the absence of the apex of the diverticulum in the intestinal lumen after EBL (Fig. 3). The procedure time was divided into three parts in accordance with a previous report11: (a) time to identify the bleeding diverticulum, (b) EBL procedure time between marking the site of bleeding with hemoclips and completing the O-band release, and (c) total procedure time (Fig. 4).
Continuous variables are presented as median (interquartile range) and were compared using the Mann-Whitney U-test. Categorical variables are presented as number (percentage) and were compared using Fisher exact test. All statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). All p-values were two-sided. A p-value <0.05 was considered statistically significant.
Of the 79 patients, 36 (45.6%) were in the C-EBL group and 43 (54.4%) were in the N-EBL group. The characteristics of the patients in both groups are shown in Table 2. There were no statistically significant differences in patient characteristics between the SRH-positive and SRH-negative groups.
The success rate of achieving initial hemostasis with EBL was 100% (36/36) in the C-EBL group and 93.0% (40/43) in the N-EBL group. In all patients in whom hemostasis was unsuccessful in the N-EBL, the mucosa of the responsible diverticulum was hardened due to diverticulitis and the band could not be applied. In these cases, additional treatments, such as EC and epinephrine injection, were performed.
The early rebleeding rate within 30 days after EBL among patients with successful initial hemostasis was 8.3% (3/36) in the C-EBL group and 17.5% (7/40) in the N-EBL group, with no significant difference between the groups (p=0.241) (Table 3). The characteristics of patients with early rebleeding are shown in Table 4.
The rate of complete inversion of the diverticula after EBL was 80.6% (29/36) in the C-EBL group and 80.0% (32/40) in the N-EBL group, with no significant difference observed between the groups (p=0.590). In terms of procedure time, no significant difference was noted between the groups in the time to identify diverticular bleeding and total procedure time. The EBL procedure time in the N-EBL group was significantly shorter than that in the C-EBL group by approximately 4 minutes (p=0.020). No complications, such as perforation or diverticulitis, were observed in either group (Table 3).
This retrospective cohort study was conducted to compare clinical outcomes of patients with CDB who were treated using the C-EBL and N-EBL devices. The N-EBL device is as safe and useful as the C-EBL device for CDB. Additionally, treatment with the N-EBL device was associated with a shorter procedure time.
The effectiveness of EBL for CDB has been widely reported. We reported that the rebleeding rate was 5.6% with EBL, which is significantly lower than the 33.3% reported with clipping.12 Furthermore, in a meta-analysis, Ishii et al.3 reported that the rate of transition to arterial embolization and surgery was lower in the ligation group than that in the coagulation or clipping group.
In contrast, the EBL method for CDB has a few disadvantages. The first is the need for reinsertion, which is time-consuming and labor-intensive for both the patient and the endoscopist. Second, there are a few reports of EBL-related adverse events such as perforation and diverticulitis due to mucosal ischemia caused by banding.13,14 Furthermore, the C-EBL device is used for esophageal varices and requires institutional approval to be used for the treatment of CDB.
In the present study, no significant difference was noted in the early rebleeding rate between the C-EBL and N-EBL groups (8.3% vs. 17.5%, p=0.241). However, the difference in the rebleeding rate was remarkable. Further studies are required to confirm the validity of our results. The characteristics of patients with rebleeding in the N-EBL group were that most cases occurred on the right side of the colon (4/7, 57.1%), and three of these seven patients were on two anti-thrombotic agents. In both groups, approximately half of the operators were experts. Rebleeding after EBL is generally known to be massive and severe in patients with CDB on the right side of the colon and those on multiple anti-thrombotic agents.15,16 These studies suggested that such cases of CDB should be carefully monitored for rebleeding even after EBL. We believe that the size and depth of the diverticulum may contribute to rebleeding, although this was not investigated in our study. Furthermore, of the 10 cases of rebleeding, five were incomplete inversion cases. In these cases, the cause was not only re-rupture of the responsible vessel in the banded diverticulum but also bleeding resulting from ulceration of the dimpling area caused by incomplete inversion (Fig. 5). In the EBL method, the mucosal inversion of the responsible diverticulum cuts off blood flow in the vasa recta, which leads to ulceration and eventually scarring. However, the dimpling area caused by incomplete inversion interferes with the complete eradication of the responsible blood vessel, resulting in rebleeding.
Therefore, we believe that it is crucial to prevent incomplete inversion to reduce the rebleeding rate. Incomplete inversion after EBL involves diverticulum-related aspects, such as size and depth and the device used. In the present study, the effect of diverticulum-related factors on incomplete inversion and rebleeding was not investigated; hence, further research is warranted. The major difference between N-EBL and C-EBL is mobility when attached to the endoscope. The diameter of the O-ring band is the same for C-EBL and N-EBL; however, the diameter of the endoscope attachment is different. The adaptive diameter of the endoscope attachment of the C-EBL was made for upper endoscopes (9.8 mm), whereas that of the N-EBL device, which was made for colonoscopes, is wider than that of the C-EBL and enables deep attachment to the endoscope because of the wide range of motion. It was assumed that when the new device was attached deeper, there would not be enough space for inversion, resulting in incomplete inversion. We believe that incomplete inversion in patients with rebleeding in N-EBL is largely due to the device and diverticulum-related aspects.
In terms of procedure time, the EBL procedure time was significantly reduced by approximately 4 minutes in the N-EBL group, although no significant difference was observed between the time to identify the diverticulum and the total procedure time. We believe that this is because the new device is able to obtain a clearer view; the N-EBL device is transparent, whereas the C-EBL device is white. In addition, as mentioned above, the new device can be deeply attached to the endoscope (Fig. 2). In most cases of CDB, the field of view is obstructed by residual feces, blood, and intestinal spasm regardless of preparation or medication used when reinserting colonoscopy with an EBL device. For these reasons, we believe that the new device can provide a better field of view, resulting in a reduction in EBL time.
In consideration of the above, it is necessary to attach the device into the scope at an appropriate depth that does not cause incomplete inversion while maintaining a clear view. In our institution, we attached the new device so that the white line on the device is aligned with the tip of the endoscope (Fig. 6A, C). We believe that this can ensure complete inversion while inserting the endoscope with a clear view; attaching deeper than this white line is likely to cause incomplete inversion (Fig. 6B, D).
The present study did not result in any complications, such as perforation after EBL, using either the C-EBL or N-EBL device. In an ex vivo study, Barker et al.17 reported that EBL was not safe for use in the small intestine and the right colon; however, it was likely to be safe for use in the left colon because the histological evaluation after EBL revealed that the band ligator involved the muscularis propria in the small intestine and right colon, whereas in the left colon, it involved the submucosa. However, most reported cases of perforation after EBL occurred in the sigmoid colon.13,14 In their case reports, the risk of diverticular perforation after EBL was considered to be delayed wound healing with long-term steroid use and mucosal hardening due to a history of diverticulitis. Conversely, animal studies have led to the widespread use of EBL in Japan. Akimaru et al.18 reported that histopathological examination revealed that the mucosal layer and muscularis propria after EBL in a pig colon were replaced with granulation tissue at the ligated sites. Moreover, we reported that perforation after EBL in a pig colon did not occur on either the right or left side, despite ligation of the muscularis propria.19 These findings suggest that EBL is a safe procedure, although further studies on the risk of perforation are recommended.
The present study had several limitations. First, this was a retrospective observational study from a single center with a relatively small sample size; therefore, unknown confounding factors could not be avoided. Second, this study did not evaluate the long-term outcomes, such as late rebleeding or diverticulitis, after 30 days. Despite these limitations, our study is the first study to compare N-EBL with C-EBL.
In conclusion, the treatment of CDB with N-EBL was found to be as safe and useful as that with C-EBL. A shorter EBL procedure time was observed in the N-EBL group. Therefore, the N-EBL device may be used as a standard treatment for patients with CDB. Further prospective studies with large sample sizes, such as randomized controlled trials, are required to confirm these results.
Notes
REFERENCES
1. Nagata N, Niikura R, Aoki T, et al. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis. 2014; 29:379–385.
2. Nakano K, Ishii N, Ikeya T, et al. Comparison of long-term outcomes between endoscopic band ligation and endoscopic clipping for colonic diverticular hemorrhage. Endosc Int Open. 2015; 3:E529–E533.
3. Ishii N, Omata F, Nagata N, et al. Effectiveness of endoscopic treatments for colonic diverticular bleeding. Gastrointest Endosc. 2018; 87:58–66.
4. Nagata N, Ishii N, Kaise M, et al. Long-term recurrent bleeding risk after endoscopic therapy for definitive colonic diverticular bleeding: band ligation versus clipping. Gastrointest Endosc. 2018; 88:841–853.e4.
5. Nagata N, Ishii N, Manabe N, et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan Gastroenterological Association. Digestion. 2019; 99(Suppl 1):1–26.
6. Witte JT. Band ligation for colonic bleeding: modification of multiband ligating devices for use with a colonoscope. Gastrointest Endosc. 2000; 52:762–765.
7. Farrell JJ, Graeme-Cook F, Kelsey PB. Treatment of bleeding colonic diverticula by endoscopic band ligation: an in-vivo and ex-vivo pilot study. Endoscopy. 2003; 35:823–829.
8. Ishii N, Itoh T, Uemura M, et al. Endoscopic band ligation with a water-jet scope for the treatment of colonic diverticular hemorrhage. Dig Endosc. 2010; 22:232–235.
9. Ishii N, Uemura M, Itoh T, et al. Endoscopic band ligation for the treatment of bleeding colonic and ileal diverticula. Endoscopy. 2010; 42(Suppl 2):E82–E83.
10. Jensen DM, Machicado GA, Jutabha R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000; 342:78–82.
11. Shimamura Y, Ishii N, Omata F, et al. Endoscopic band ligation for colonic diverticular bleeding: possibility of standardization. Endosc Int Open. 2016; 4:E233–E237.
12. Setoyama T, Ishii N, Fujita Y. Enodoscopic band ligation (EBL) is superior to endoscopic clipping for the treatment of colonic diverticular hemorrhage. Surg Endosc. 2011; 25:3574–3578.
13. Sato Y, Yasuda H, Fukuoka A, et al. Delayed perforation after endoscopic band ligation for colonic diverticular hemorrhage. Clin J Gastroenterol. 2020; 13:6–10.
14. Takahashi S, Inaba T, Tanaka N. Delayed perforation after endoscopic band ligation for treatment of colonic diverticular bleeding. Dig Endosc. 2016; 28:484.
15. Gilshtein H, Kluger Y, Khoury A, et al. Massive and recurrent diverticular hemorrhage, risk factors and treatment. Int J Surg. 2016; 33 Pt A:136–139.
16. Nagata N, Niikura R, Aoki T, et al. Colonic diverticular hemorrhage associated with the use of nonsteroidal anti-inflammatory drugs, low-dose aspirin, antiplatelet drugs, and dual therapy. J Gastroenterol Hepatol. 2014; 29:1786–1793.
17. Barker KB, Arnold HL, Fillman EP, et al. Safety of band ligator use in the small bowel and the colon. Gastrointest Endosc. 2005; 62:224–227.
18. Akimaru K, Suzuki H, Tsuruta H, et al. Eversion and ligation of a diverticulum: report of an inspirational case and subsequent animal study. J Nippon Med Sch. 2008; 75:157–161.
19. Shiratori Y, Ikeya T, Suzuki K, et al. Comparison of endoscopic band ligation devices used for colonic diverticular bleeding: in vivo animal study. JGH Open. 2021; 5:50–55.
Fig. 1.
(A) The appearance of a new endoscopic band ligation device. (B) The appearance of a conventional endoscopic band ligation device.
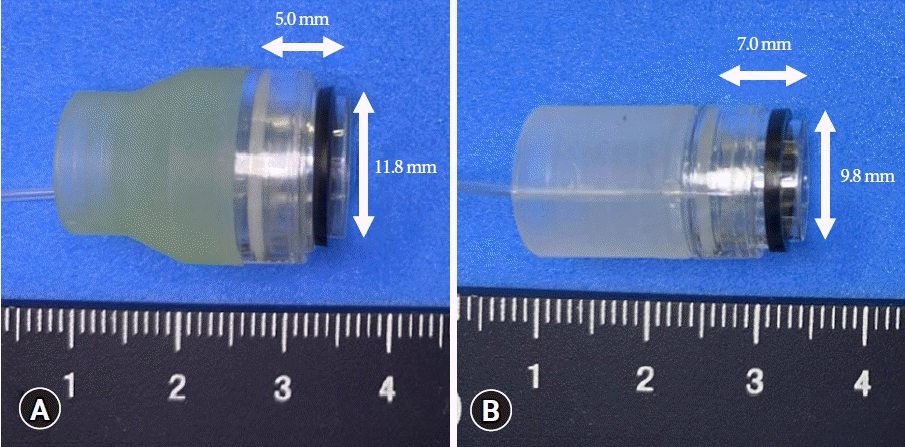
Fig. 2.
(A) The view of the new endoscopic band ligation device. (B) The view of the conventional endoscopic band ligation device.
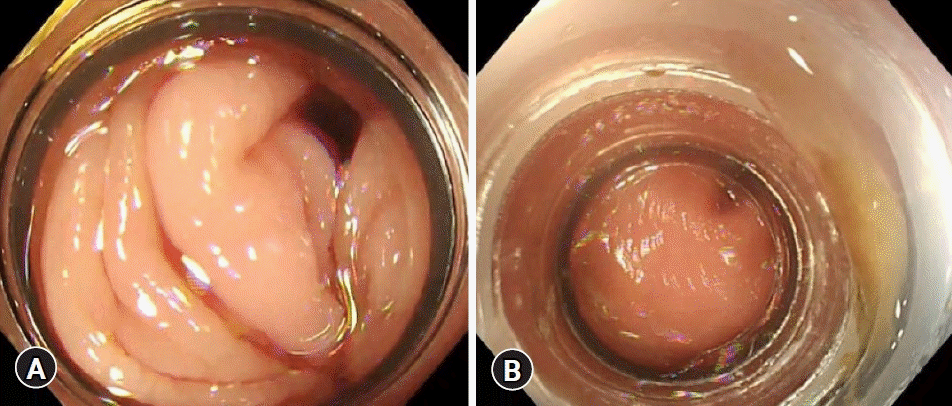
Fig. 5.
(A) Incomplete inversion of the diverticulum after endoscopic band ligation. (B) The ulceration of dimpling area caused a few days after endoscopic band ligation.
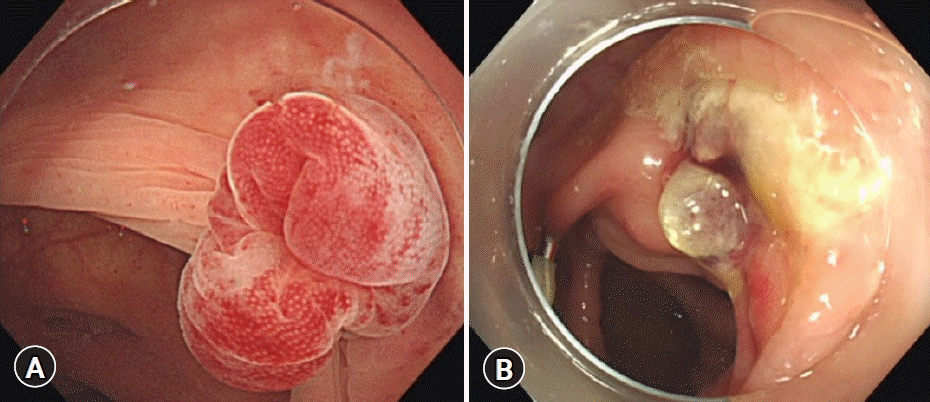
Fig. 6.
The recommended method for device attachment. (A, C) The tip of the endoscope (yellow triangle) is positioned on the white line of the hood (white triangle), which can achieve complete inversion. (B, D) If the tip of the endoscope is located beyond the white line of hood, incomplete inversion may result.
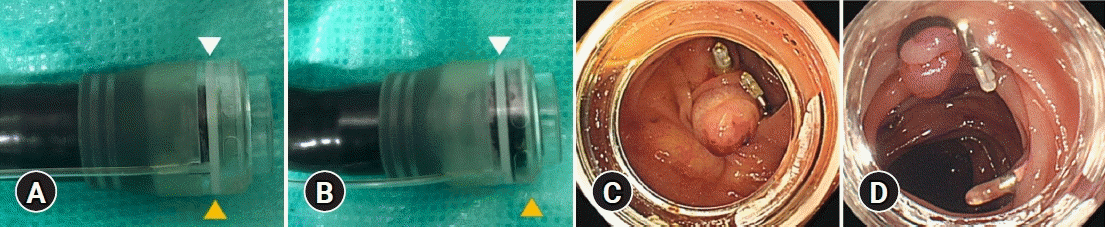
Table 1.
Features of the endoscopic band ligation devices
Table 2.
Patient characteristics
Table 3.
Outcome measurements
Table 4.
Patients with rebleeding
SRH, stigmata of recent hemorrhage; C, conventional endoscopic band ligation device; S/C, sigmoid colon; AB, active bleeding; HT, hypertension; A/C, ascending colon; IHD, ischemic heart disease; EBL, endoscopic band ligation; N, new endoscopic band ligation device; DM, diabetes mellitus; CKD, chronic kidney disease; T/C, transverse colon; NBVV, non-bleeding visible vessel; AC, adherent clot.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download