Abstract
Background/Aims
The efficacy of propofol in gastrointestinal endoscopy for patients with midazolam-induced paradoxical reactions remains unclarified. This study aimed to compare the efficacy and safety of propofol-based sedation in patients who previously experienced paradoxical reactions.
Methods
This was a prospective, single-blinded, randomized controlled pilot study. Participants with a history of paradoxical reactions to midazolam during a previous esophagogastroduodenoscopy were recruited and randomly assigned to group I (propofol monosedation) or group II (combination of propofol and midazolam). The primary endpoint was the occurrence of a paradoxical reaction.
Results
A total of 30 participants (mean age, 54.7±12.6 years; male, 19/30) were randomly assigned to group I (n=16) or group II (n=14). There were no paradoxical reactions in group I, but there were two in group II, without a significant difference (p=0.209). The mean dose of propofol was higher in group I than in group II (p=0.002). Meanwhile, the procedure and recovery times did not differ between groups.
Midazolam, a short-acting hypnotic-sedative benzodiazepine, is relatively safe for endoscopic sedation and is widely used in gastrointestinal (GI) endoscopy facilities. Paradoxical reactions are well-known adverse effects following midazolam administration [1]. Since paradoxical reactions cause excessive excitement, anxiety, and violent behavior, they lead to safety and quality issues during endoscopic procedures. According to current guidelines, safety is regarded as a key performance measure for improving GI endoscopy quality [2,3]. Accordingly, a dose reduction in midazolam with opioid analgesics has been positively considered for paradoxical reactions [4,5]. Additionally, the administration of flumazenil, an antidote for midazolam, can completely and effectively reverse midazolam-induced paradoxical reactions [6,7]. However, flumazenil may induce withdrawal symptoms, such as confusion and agitation, in patients with benzodiazepine dependence [8].
Propofol is a short-acting anesthetic, which can be used for procedural sedation. It has an onset of action of less than one minute and a short duration of three to 10 minutes. The endoscopist-directed administration of propofol has been shown to have a low risk of serious complications and is cost-effective [9,10]. Balanced propofol sedation (BPS), which targets moderate sedation using propofol in combination with opiates and benzodiazepines, has better patient satisfaction than standard sedation with midazolam plus opiates during endoscopy [11]. Moreover, propofol monosedation resulted in faster recovery and less time in the endoscopy unit than BPS for both esophagogastroduodenoscopy (EGD) and colonoscopy procedures [12]. Additionally, propofol-based endoscopic sedation has proven its effectiveness and safety for pediatric and therapeutic endoscopy [13,14]. However, the efficacy of propofol as a sedative drug in patients with midazolam-induced paradoxical reactions during endoscopy has yet to be clarified. Given that patients administered with propofol have lesser discomfort, need for adjustment, and memory of the procedure compared with midazolam [12], the positive effects of propofol use are expected in patients with paradoxical reactions who frequently show anxiety and restlessness. This pilot study aimed to evaluate the outcome of propofol-based sedation in patients who experienced paradoxical reactions to midazolam and compare the safety of propofol monosedation and propofol in combination with midazolam (BPS) during sedative EGD.
This was a prospective, single-blinded, randomized controlled study conducted at Dongguk University Ilsan Hospital, Incheon St. Mary’s Hospital, and Yeungnam University Hospital in South Korea. Subjects who experienced a paradoxical reaction to midazolam on a previous EGD and were planning to undergo EGD were eligible for the study. Paradoxical reactions were defined as increased talkativeness, emotional release, excitement, excessive movement, hostility, and rage, during and after the procedures [6], and were reported as adverse drug reactions. We reviewed their endoscopic reports at the time for patients who had previously reported adverse drug reactions to midazolam. The following subjects were excluded in the study: younger than 19 years old, with an American Society of Anesthesiologists (ASA) class of IV or higher, or preference of a non-sedative EGD. The present study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (IRB no. 2018-02-011) and registered at ClinicalTrials.gov (identifier NCT04072328). Informed consent was obtained from all participants enrolled in the study.
The participants who agreed to enroll were randomly assigned to group I (propofol monosedation) or group II (BPS, combination of propofol and midazolam). Sequentially numbered sealed envelopes with computer-generated random allocations at a ratio of 1:1 in balanced blocks of four were used for the randomization process. An independent hospital staff member created random numbers, which were kept in sealed envelopes. The investigators were aware of the group allocations, but the participants were unaware of their group assignments. This was a pilot study with no previous references. The sample size was calculated with an alpha error of 0.05 and a statistical power of 0.8, assuming that paradoxical reactions would occur in approximately 20% of group I and 70% of the group II. Finally, the sample size was estimated to be 30 subjects (15 in each group).
All participants were instructed to fast overnight and examined by experienced endoscopists using a video EGD (Olympus GIF-H290 or GIF-H260; Olympus Optical Co., Tokyo, Japan). The administered dose of propofol or midazolam was determined according to a unified protocol (dose adjusted to patient age, weight, and comorbidity) [15]. For group I, intravenous propofol (Presofol MCT; Fresenius Kabi Austria GmbH, Graz, Austria) was administrated at a dose of 0.5 mg/kg, followed by 10-20 mg of propofol every 30 seconds based on the patient’s sedation level. For group II, intravenous midazolam (Midazolam; Bukwang Pharm Co., Seoul, South Korea) was administered at a dose of 0.06 mg/kg plus propofol at a dose of 10-20 mg, followed by 10-20 mg of propofol every 30 seconds based on the patient’s sedation level. The intended level of sedation was moderate sedation (i.e., conscious sedation), a condition in which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation without the need for interventions to maintain a patent airway and cardiovascular function [15,16]. For sedation monitoring, blood pressure, respiratory rate, pulse rate, and oxygen saturation were checked at least 15 minutes before the procedure. Moreover, we monitored the above four parameters and level of consciousness every two minutes during the procedure and every 15 minutes during recovery. The modified Aldrete score was graded from 0-2 for the following five categories: oxygenation, respiration, circulation, consciousness, and activity. A score of ≥ 9 out of 10 was the standard for discharge [17].
The primary endpoint of this study was the occurrence of a paradoxical reaction during the procedure. The following were analyzed as secondary outcomes: the dose of sedative drugs, adverse events related to sedation, the incompletion rate, the endoscopic procedure time, recovery time, and the modified Aldrete score. The development of an adverse event was defined as a decrease in oxygen saturation of ≤90% or a ≥20% change in systolic blood pressure, respiratory rate, or pulse rate compared to the baseline.
The objective of this pilot study was to assess the efficacy and safety of propofol-based sedation protocols in patients with a history of paradoxical reactions. Chi-square or Fisher’s exact test was used to compare categorical variables between groups. Consequently, the independent sample t-test or the Mann-Whitney U test was used to compare continuous variables such as sedative dose or procedure time. Two-sided P values of <0.05 were considered statistically significant. All statistical analyses were conducted using SPSS Statistics (version 19.0; IBM, Armonk, NY, USA).
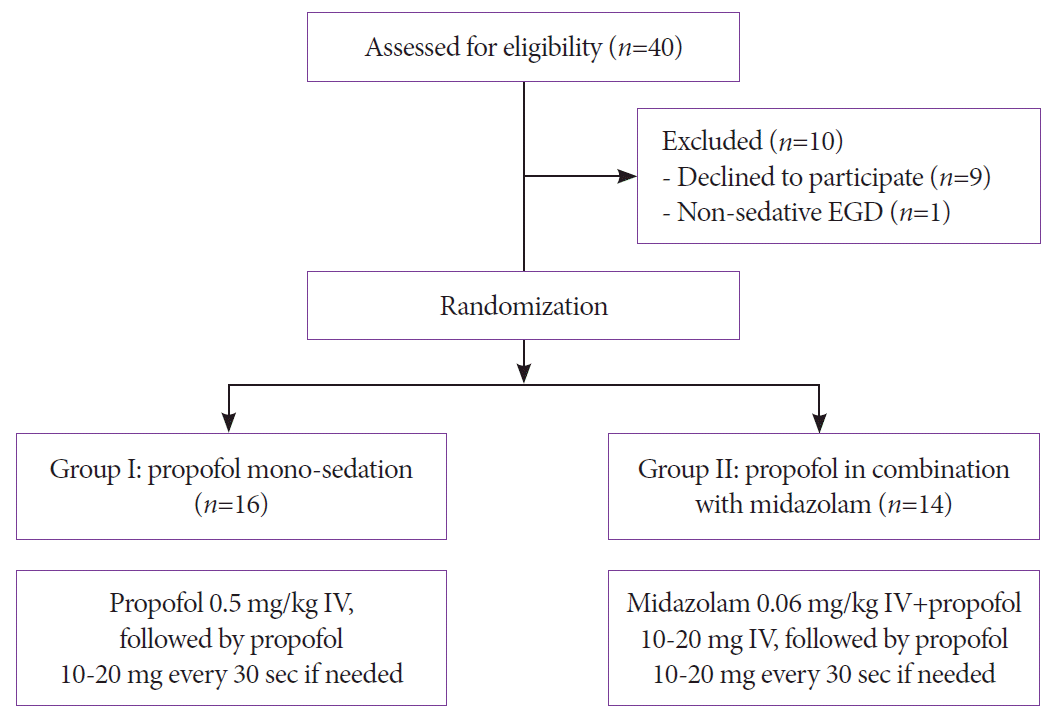
Initially, 40 participants were eligible for the trial. After excluding 10 participants, a total of 30 participants were randomly assigned to group I (propofol monosedation, n=16) or group II (BPS, n=14) from October 2018 to April 2020 (Fig. 1). The mean age was 54.7±12.6 years (range, 25-86), while the male-to-female ratio was 19:11. There were no significant differences in age and sex distribution, body mass index, smoking and drinking behaviors, ASA scores, baseline blood pressure, and pulse rate between the groups (Table 1). Additionally, patient comorbidities and endoscopist distributions were not different.
Table 2 shows the results of the main outcome comparisons between the two groups. There were no cases of paradoxical reactions in group I, but there were two (14.3%) in group II, without a significant difference (p=0.209). The mean dose of administered midazolam was 3.2±0.8 mg in group II. The mean dose of propofol was significantly higher in group I than in group II (31.9±8.9 vs. 18.2±12.3 mg; p=0.002). Meanwhile, the procedure and recovery times were not different between the groups, but the recovery time was shorter in group I. The mean modified Aldrete score was 10.0±0 and 9.7±0.7 in groups I and II, respectively (p=0.124).
This study is the first to evaluate the effect of propofol-based sedation on midazolam-induced paradoxical reactions during sedative endoscopy. Only two out of 30 patients exhibited a paradoxical reaction during the scheduled EGD. There were no serious adverse events or incomplete examinations in both the propofol monosedation and propofol combined with midazolam groups. Despite being a pilot study, it has clinical significance as a prospective study confirming whether paradoxical reactions to midazolam can be reduced by propofol-based sedation.
Globally, the incidence of midazolam-induced paradoxical reactions during conventional endoscopy has been reported within 1 to 10% [1,18,19], which may further increase with time-consuming therapeutic endoscopy [20]. The occurrence of a paradoxical reaction can cause a safety issue and interference with the procedures, resulting in repeat examinations and additional costs. Moreover, experiencing a paradoxical reaction may be a factor for patients to avoid a future endoscopy.
Flumazenil administration can reverse a paradoxical reaction; however, it is not a prophylactic agent, and it may cause serious side effects. On the other hand, propofol sedation is safe and effective for pediatric EGD. A previous study reported that the midazolam plus meperidine group required restraint more often than patients in the propofol group [13]. In addition, BPS and propofol monosedation showed comparable and satisfactory outcomes in sedation safety and endoscopic procedures, such as colonoscopy and endoscopic retrograde cholangiopancreatography [14,21,22]. Moreover, sedation using propofol was safe and highly satisfactory in patients with gastric cancer who underwent endoscopic submucosal dissection [23]. These findings indicate that propofol-based sedation may be helpful for patients who are vulnerable to endoscopy or relatively prolonged, difficult procedures.
The patients with paradoxical reactions tended to be more sensitive to endoscopy and generally more difficult to induce an adequate sedation level than those without paradoxical reactions. Prospective surveillance studies have shown that propofol is highly satisfactory for both patients and endoscopists [24,25]. Thus, the use of propofol-based sedatives may be reasonable for patients who had a paradoxical reaction. Meanwhile, the most common adverse event following propofol administration is hypotension, which is more common in critically ill patients [26]. Considering that propofol has a narrow margin of safety and no antagonists [25], precautions should be taken, especially when administering it to critically ill or elderly patients.
Our study showed comparable results between propofol monosedation and BPS in patients with a history of paradoxical reactions to midazolam. However, paradoxical events were more common, and the recovery time was likely to be longer in the BPS group. Given that this study was underpowered due to its pilot study nature, these results provide valuable insight for planning a large-scale trial with sufficient discrimination power. Both propofol monosedation and BPS can be safely applied in patients with a history of paradoxical reactions to midazolam. Nonetheless, caution must be exercised because paradoxical reaction again can happen in the BPS group in which midazolam was readministered.
Generally, patients who previously had a paradoxical response seemed to recur on the next endoscopy. However, only two patients showed paradoxical reactions in this study. We considered this to be the effect of proper sedation via propofol and dose reduction of midazolam. The incidence of midazolam-induced paradoxical reactions is related to the total dose of midazolam administered [20]. In the present study, a paradoxical reaction was observed only in the group administered with the combination of propofol and midazolam and in two out of three patients whose midazolam dose exceeded 5 mg. Meanwhile, a previous study showed that paradoxical reactions were observed in 16% of propofol-induced sedations and were associated with young age and anxiety related to excessive worry, health concerns, or sleeping problems [27]. The two patients in our study who had paradoxical reactions to propofol-based sedation were 25 and 60 years old. They did not present any anxiety or psychiatric problems before sedation. Further largescale studies are required to determine which characteristics are related to a paradoxical reaction to sedatives.
This study has several limitations. First, we could not identify any significant differences in the outcomes because this planned pilot study was underpowered. More significant outcomes are expected if a large-scale trial is conducted based on the outcomes of this study. Second, since the endoscopists were not blinded to the group to which the patient belonged, the outcome variables may have been affected depending on the endoscopist’s intention. Therefore, to minimize the examiner’s subjectivity, we followed a documented protocol for sedative use and determined whether a paradoxical reaction or adverse event occurred based on defined criteria.
In conclusion, this prospective study showed the safety and efficacy of propofol-based sedation for patients with paradoxical reactions, which did not differ between the groups administered with propofol alone and propofol in combination with midazolam. Thus, propofol-based sedation may be considered for subsequent endoscopy in patients with a history of midazolam-induced paradoxical reaction. This study provides clinical evidence that supports the practical use of propofol-based sedation for such patients and suggests the need for future multi-center studies.
Notes
REFERENCES
1. Yi SY, Shin JE. Midazolam for patients undergoing upper gastrointestinal endoscopy: a prospective, single-blind and randomized study to determine the appropriate amount and time of initiation of endoscopy. J Gastroenterol Hepatol. 2005; 20:1873–1879.
2. Valori R, Cortas G, de Lange T, et al. Performance measures for endoscopy services: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J. 2019; 7:21–44.
3. Lee JK, Lee YJ, Cho JH, et al. Updates on the sedation for gastrointestinal endoscopy. Clin Endosc. 2019; 52:451–457.
4. Tae CH, Kang KJ, Min BH, et al. Paradoxical reaction to midazolam in patients undergoing endoscopy under sedation: incidence, risk factors and the effect of flumazenil. Dig Liver Dis. 2014; 46:710–715.
5. Hong GW, Lee JK, Lee JH, et al. Comparison of fentanyl versus meperidine in combination with midazolam for sedative colonoscopy in Korea. Clin Endosc. 2020; 53:562–567.
6. Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004; 24:1177–1185.
7. Weinbroum AA, Szold O, Ogorek D, Flaishon R. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol. 2001; 18:789–797.
8. Honan VJ. Paradoxical reaction to midazolam and control with flumazenil. Gastrointest Endosc. 1994; 40:86–88.
9. Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009; 137:1229–1237. quiz 1518-1519.
10. Lee JK, Jang DK, Kim WH, Kim JW, Jang BI. [Safety of non-anesthesiologist administration of propofol for gastrointestinal endoscopy]. Korean J Gastroenterol. 2017; 69:55–58.
11. Levitzky BE, Lopez R, Dumot JA, Vargo JJ. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy. 2012; 44:13–20.
12. Poulos JE, Kalogerinis PT, Caudle JN. Propofol compared with combination propofol or midazolam/fentanyl for endoscopy in a community setting. AANA J. 2013; 81:31–36.
13. Khoshoo V, Thoppil D, Landry L, Brown S, Ross G. Propofol versus midazolam plus meperidine for sedation during ambulatory esophagogastroduodenoscopy. J Pediatr Gastroenterol Nutr. 2003; 37:146–149.
14. Lee TH, Lee CK, Park SH, et al. Balanced propofol sedation versus propofol monosedation in therapeutic pancreaticobiliary endoscopic procedures. Dig Dis Sci. 2012; 57:2113–2121.
15. Dumonceau JM, Riphaus A, Aparicio JR, et al. European society of gastrointestinal endoscopy, european society of gastroenterology and endoscopy nurses and associates, and the european society of anaesthesiology guideline: non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010; 42:960–974.
16. Harris ZP, Liu J, Saltzman JR. Quality assurance in the endoscopy suite: sedation and monitoring. Gastrointest Endosc Clin N Am. 2016; 26:553–562.
17. McGrath B, Chung F. Postoperative recovery and discharge. Anesthesiol Clin North Am. 2003; 21:367–386.
18. Golparvar M, Saghaei M, Sajedi P, Razavi SS. Paradoxical reaction following intravenous midazolam premedication in pediatric patients - a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth. 2004; 14:924–930.
19. Massanari M, Novitsky J, Reinstein LJ. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr (Phila). 1997; 36:681–684.
20. Terui T, Inomata M. Administration of additional analgesics can decrease the incidence of paradoxical reactions in patients under benzodiazepine-induced sedation during endoscopic transpapillary procedures: prospective randomized controlled trial. Dig Endosc. 2013; 25:53–59.
21. Shin S, Oh TG, Chung MJ, et al. Conventional versus analgesia-oriented combination sedation on recovery profiles and satisfaction after ERCP: a randomized trial. PLoS One. 2015; 10:e0138422.
22. VanNatta ME, Rex DK. Propofol alone titrated to deep sedation versus propofol in combination with opioids and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol. 2006; 101:2209–2217.
23. Kiriyama S, Naitoh H, Kuwano H. Propofol sedation during endoscopic treatment for early gastric cancer compared to midazolam. World J Gastroenterol. 2014; 20:11985–11990.
24. Kanno Y, Ohira T, Harada Y, et al. Safety and recipient satisfaction of propofol sedation in outpatient endoscopy: a 24-hour prospective investigation using a questionnaire survey. Clin Endosc. 2021; 54:340–347.
25. Cohen LB, Wecsler JS, Gaetano JN, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006; 101:967–974.
26. Park CH, Han DS, Jeong JY, et al. Outcomes of propofol sedation during emergency endoscopy performed for upper gastrointestinal bleeding. Dig Dis Sci. 2016; 61:825–834.
27. Lee SH, Lee GM, Lee DR, Lee JU. Factors related to paradoxical reactions during propofol-induced sedated endoscopy. Scand J Gastroenterol. 2019; 54:371–376.
Table 1.
Baseline Characteristics Compared between Patients in Group I and Group II
| Variables | Group Ia)(propofol alone) (n=16) | Group IIa)(combination) (n=14) | p value |
|---|---|---|---|
| Age (years), mean±SD | 52.1±10.8 | 57.7±14.3 | 0.227 |
| M:F ratio | 9:7 | 10:4 | 0.389 |
| BMI ≥25 kg/m2, n (%) | 5 (31.3) | 2 (14.3) | 0.399 |
| Smoking, n (%) | 6 (37.5) | 2 (14.3) | 0.226 |
| Alcohol, n (%) | 8 (50.0) | 5 (35.7) | 0.431 |
| ASA, n (%) | 0.630 | ||
| I | 5 (31.3) | 5 (35.7) | |
| II | 10 (62.5) | 9 (64.3) | |
| III | 1 (6.3) | 0 | |
| Hypertension, n (%) | 5 (31.3) | 7 (50.0) | 0.296 |
| Diabetes, n (%) | 3 (18.8) | 4 (28.6) | 0.675 |
| Baseline BP ≥150/90 (mmHg), n (%) | 2 (12.5) | 2 (14.3) | 0.886 |
| Baseline PR ≥100/min, n (%) | 4 (25.0) | 4 (28.6) | 0.825 |
| Endoscopist, n | |||
| Experienced | 10 | 9 | 0.919 |
| Trainee | 6 | 5 |
Table 2.
Comparison of Outcomes According to Propofol-Based Sedation Protocols
| Variables | Group Ia)(propofol alone) (n=16) | Group IIa)(combination) (n=14) | p value |
|---|---|---|---|
| Paradoxical reaction, n (%) | 0 | 2 (14.3) | 0.209 |
| Dose of sedative drugs (mg), mean±SD | |||
| Midazolam | 0 | 3.2±0.8 | N/A |
| Propofol | 31.9±8.9 | 18.2±12.3 | 0.002 |
| Procedure time (min), mean±SD | 4.4±1.9 | 3.9±1.3 | 0.343 |
| Recovery time (min), mean±SD | 31.4±18.1 | 37.9±21.5 | 0.378 |
| Modified Aldrete score,b) mean±SD | 10.0±0.0 | 9.7±0.7 | 0.124 |
| Incomplete examination, n (%) | 0 | 0 | N/A |
| Adverse event, c) n (%) | 0 | 0 | N/A |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download