Abstract
Background/Aims
Methods
Results
Notes
Conflicts of Interest
Juan E. Corral: Travel grant from AbbVie, Inc.; Minor food and beverage from Boston Scientific and Cook Medical.
Emmanuel Coronel: Consulting for Boston Scientific.
Michael B. Wallace: Consulting for Virgo Inc, Cosmo/Aries Pharmaceuticals, Anx Robotica (2019), Covidien, and GI Supply; Research grants from Fujifilm, Boston Scientific, Olympus, Medtronic, Ninepoint Medical, Cosmo/ Aries Pharmaceuticals; Stock options from Virgo Inc; Consulting on behalf of Mayo Clinic, GI Supply (2018), Endokey, Endostart, Boston Scientific, and Microtek; General payments/minor food and beverage from Synergy Pharmaceuticals, Boston Scientific, and Cook Medical.
The authors have no potential conflicts of interest.
Author Contributions
Conceptualization: Juan E. Corral
Data curation: Do Han Kim, Emmanuel Coronel, Paul T. Kröner
Formal analysis: DHK, EC, PTK
Funding acquisition
Investigation: DHK, EC, PTK
Methodology: JEC
Project administration: JEC
Resources: JEC
Supervision: JEC
Validation: Herbert C. Wolfsen, Michael B. Wallace
Visualization: Somashekar G Krishna, MBW
Writing-original draft: JEC
Writing-review & editing: DHK, HCW, MBW
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
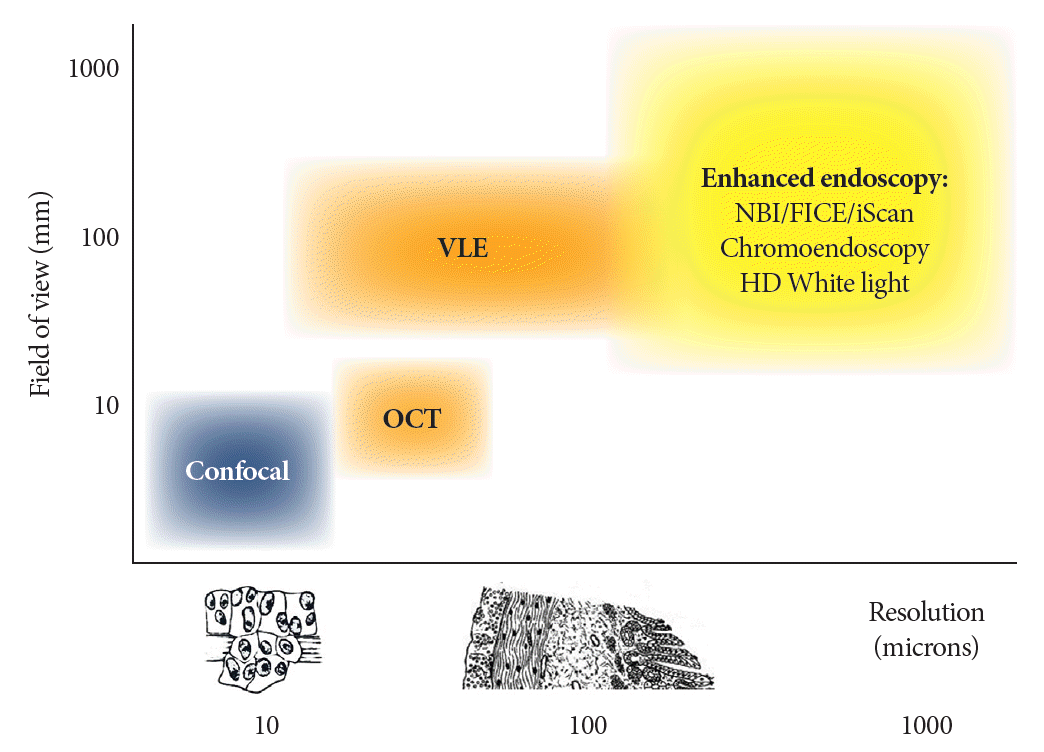
Fig. 2.
Fig. 3.
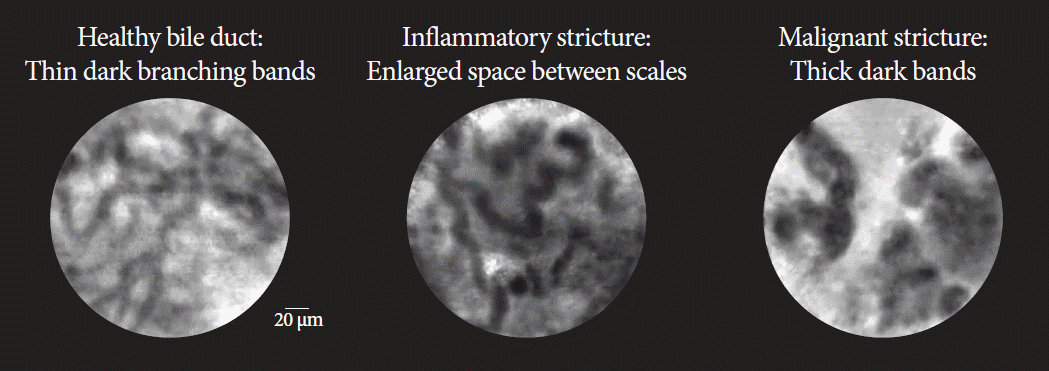
Fig. 4.
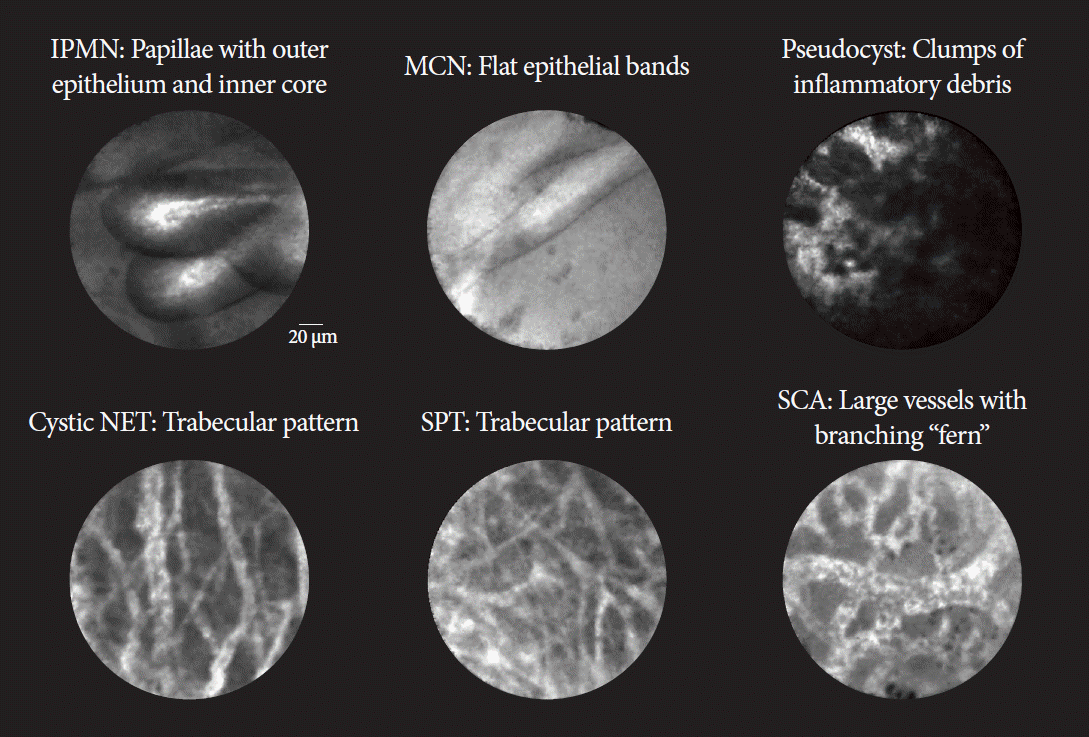
Table 1.
| Healthy bile duct | Inflammatory stricture | Malignant stricture | |
|---|---|---|---|
| Collagen fibrils | Reticular network of thin dark branching bands (<20 μm) | Dark granular pattern in scales Thickened reticular structures | Thick dark bands (>40 μm) |
| Background | Light grey | Roughness aspect | Dark clumps |
| Vessels | Thin white bands (<20 μm) | Vascular congestion | Thick white bands (>20 μm) |
| Epithelium | Enlarged space between scales Increased inter-glandular space | Epithelium visualized (villi, glands) | |
| Additional featuresa) | Fluorescein leakage |
Table 2.
|
Biliary strictures |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Aim | Study design | Sensitivity | Specificity | Accuracy | Pancreatitis | Comments |
| Meining et al. (2011) [24], n=86a) | Diagnosis of cholangiocarcinoma. | Prospective multicenter | 98% | 67% | 81% | 0% | 3 pancreatic strictures included |
| Heif et al. (2013) [30], n=15 | Dominant stricture in PSC. | Case series single center | 100% | 61% | 66% | 0% | PSC only |
| Caillol et al. (2013) [26], n=60 | Standardize image interpretation | Retrospective image review | 96% | 76% | 85% | NA | Focused on image standardization |
| EMID (2015) [31], n=61a) | Malignant vs. benign strictures | Prospective single center | 100% | 71% | 93% | NA | Added EUS-guided biopsies |
| FOCUS (2015) [32], n=112 | Malignant vs. benign strictures | Prospective multicenter | 89% | 71% | 82% | 0% | Excluded patients with PSC |
| Fugazza et al. (2016) [18], 10 studies, n=494 | Malignant vs. benign strictures | Systematic review and meta-analysis | Pooled 90% | Pooled 72% | Pooled 81% | NA | Also reviewed Barrett’s, gastric and colorectal cancer |
| Liu et al. (2016) [17], 8 studies, n=280 | Malignant vs. benign strictures | Systematic review and meta-analysis | Pooled 90% | Pooled 75% | Pooled 82% | NA | |
| Dubow et al. (2018) [33], n=97 | Malignant vs. benign strictures | Retrospective single center | 83% | 93% | 90% | NA | Prior ERCP sampling and imaging negative |
| Koda et al. (2021) [34], n=7 | Malignant vs. benign strictures | Case series single center | GastroFlexTM 100% | 0% (0/3) | 57.1% | NA | |
| CholangioFlexTM 75% | 66.7% | 71.4% | |||||
| AlveoFlexTM 75% | 33.3% | 57.1% | |||||
Table 3.
|
Pancreatic cysts |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Aim | Study design | Sensitivity | Specificity | Accuracy | Pancreatitis | Comments |
| INSPECT (2013), [35] n=57 | SCA and pseudocyst vs. other cysts | Prospective multicenter | 59% | 100% | 71% | 3% | Early phase defining diagnostic criteria |
| DETECT (2015), [36] n=29 | Mucinous vs. other cysts | Prospective single center | 80% | 100% | 89% | 7% | Combination with cystoscopy yields 100% accuracy |
| Fugazza et al. (2016), [18] 5 studies, n=163 | Malignancy in pancreatic cysts | Systematic review and meta-analysis | Pooled 68% | Pooled 90% | Pooled 79% | NA | Also reviewed Barrett’s, gastric and colorectal cancer |
| CONTACT (2015), [37] n=43 (included 31) | SCA vs. other cysts | Prospective multicenter | SCA 95% | 100% | 99% | 2% | Superior than combination of CEA and cytology analysis |
| Indeterminate mucinous 95% | 100% | 97% | |||||
| NET 100% | 95% | 96% | |||||
| Premalignant cyst 96% | 95% | 96% | |||||
| INDEX (2019), [38] n=144 | Mucinous vs. non mucinous pancreatic cysts | Prospective single center | 98% | 94% | 97% | 3% | Superior than combination of CEA and cytology analysis |
| CONCYST (2019), [39] n=67 (included 56) | Indeterminate pancreatic cyst | Prospective multicenter | All indeterminate cysts 80% | NA | 77% | 0% | Correlation with pathology and experts was good. Image acquisition took <10 min |
| IPMN 90% | 87% | ||||||
| Ductal adenocarcinoma 100% | 100% | ||||||
| SCA 56% | 38% | ||||||
| Pseudocysts 67% | 67% | ||||||
| Krishna et al. (2020), [38,40] n=26 | Identify dysplasia in IPMN | INDEX post-hoc analysis | Papillary epithelial width 88% | 100% | 85% | 3% | Allow risk stratification of IPMN |
| Papillary epithelial darkness 88% | 100% | 84% | |||||
| Hao et al. (2020), [41] n=122 | Solid and cystic pancreatic lesions | Prospective single center | All cysts 94% | 98% | 97% | 5% | |
| SCA 89% | 100% | 97% | |||||
| MCN 87% | 98% | 94% | |||||
| IPMN 97% | 100% | 99% | |||||
| Facciorusso et al. (2020), [15] 10 studies, n=536 | Pancreatic cystic lesions | Systematic review and meta-analysis | Pooled 82% | Pooled 97% | 89% | 0% | Mean procedure duration of 6 mins |
| Konjeti et al. (2020), [19] 7 studies, n=324 | Pancreatic cystic lesions | Systematic review and meta-analysis | Pooled 85% | 99% | 99% | 1% | High heterogeneity among studies |
| Chin et al. (2021), [42] 42 studies, n=519 | Pancreatic cystic lesions | Systematic review | 2.6% | No meta-analysis performed | |||
| Pancreatic parenchyma and solid lesions | |||||||
| Giovannini et al. (2016), [43] n=40 | Solid pancreatic lesions, compared to pathology | Prospective multicenter. Part of CONTACT | Ductal adenocarcinoma 77% | 100% | 85% | NA | First description of adenocarcinoma, NET and chronic pancreatitis |
| Chronic pancreatitis 50% | 100% | 91% | |||||
| NET 100% | 97% | 97% | |||||
| Hao et al. (2020), [41] n=50 | Solid and cystic pancreatic lesions | Prospective single center | Ductal adenocarcinoma 90% | 89% | 90% | 5% | First description of AIP and tuberculosis |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download