Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) of gastric tumors in the mid-to-upper stomach is a technically challenging procedure. This study compared the therapeutic outcomes and adverse events of ESD of tumors in the mid-to-upper stomach performed under general anesthesia (GA) or monitored anesthesia care (MAC).
Methods
Between 2012 and 2018, 674 patients underwent ESD for gastric tumors in the midbody, high body, fundus, or cardia (100 patients received GA; 574 received MAC). The outcomes of the propensity score (PS)-matched (1:1) patients receiving either GA or MAC were analyzed.
Results
The PS matching identified 94 patients who received GA and 94 patients who received MAC. Both groups showed high rates ofen bloc resection (GA, 95.7%; MAC, 97.9%; p=0.68) and complete resection (GA, 81.9%; MAC, 84.0%; p=0.14). There were no significant differences between the rates of adverse events (GA, 16.0%; MAC, 8.5%; p=0.18) in the anesthetic groups. Logistic regression analysis indicated that the method of anesthesia did not affect the rates of complete resection or adverse events.
Endoscopic submucosal dissection (ESD) is widely used because it can achieve a higher rate of complete gastric tumor resection than endoscopic mucosal resection [1,2]. Additionally, ESD is less invasive and can result in a better quality of life than surgical gastrectomy, if indicated [3]. However, it requires sophisticated endoscopic techniques. Thus, the adverse events, such as bleeding and perforation and clinical outcomes following en bloc resection depend on the technical skill of the endoscopists, especially in difficult ESD cases. A previous study demonstrated that tumors located in the upper third of the stomach are associated with longer procedure duration and higher frequencies of incomplete resection than those located in other stomach areas [3]. Other studies have shown that the mid to upper stomach region has more vessels and is more likely to have early gastric cancers (EGCs) with submucosal invasion than other areas of the stomach [4,5]. Therefore, ESD procedures for tumors located in the mid to upper stomach require high levels of endoscopic technical skill.
ESD also requires minimal patient movement during the procedure [6,7]. Therefore, previous studies have discussed the appropriate sedation methods for use during ESD. These studies have compared different sedatives, such as midazolam and propofol, or traditional sedation (administered by endoscopists) with sedatives and anesthetic care via intubation [1,2,6-9]. Some studies have also reported ESD outcomes in patients under general anesthesia (GA) [1,10].
The present study was based on the premise that ESD involving the mid-to-upper stomach is more difficult than that involving the lower part of the stomach. Hence, we assumed that resections in the mid-to-upper stomach are less likely to be complete and more likely to be associated with adverse events than those in the lower stomach. ESDs conducted under GA with intubation are more effective and safer than procedures involving traditional sedation administered by endoscopists [1,2,10]. However, a direct comparison between monitored anesthesia care (MAC) without intubation and GA for ESD has not been previously reported. Thus, this study compared the therapeutic outcomes and adverse events following ESD of tumors located in the mid-to-upper stomach under GA and MAC.
This retrospective study was performed at the Samsung Medical Center, Seoul, South Korea. Between January 2012 and December 2018, 3,760 patients underwent ESD for gastric tumors under GA or MAC. The tumors were located in the mid-to-upper stomach, including the midbody, high body, fundus, and cardia. Histologically confirmed EGCs, adenomas, and neuroendocrine tumors (NETs) were included based on the final pathologic reports from the ESD specimens (Fig. 1). Patients with a prior history of esophagectomy for esophageal cancer were excluded. Patients who underwent combined ESD or gastrectomy were excluded. The study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center. Because this study was based on a retrospective analysis of existing clinical data, the requirement for informed patient consent was waived by the Institutional Review Board (No. 2019-09-015-001).
Five gastroenterologists performed all gastric ESD procedures using standard techniques. First, a circumferential mark was made around the lesion using a needle knife or a dual knife. Thereafter, fluid (normal saline [100 mL], epinephrine [1 mL], and 0.8% indigo carmine [0.1 mL]) was injected into the submucosal layer. A circumferential mucosal precut was made, and the submucosal layer was dissected using various types of knives, such as the IT2 knife or dual knife. Endoscopic hemostasis was performed simultaneously whenever bleeding was observed (Fig. 2).
The ESD procedure was performed under GA with endotracheal intubation or MAC without intubation. GA was induced with rocuronium, remifentanil, and propofol and maintained with propofol and remifentanil. MAC was induced with midazolam and maintained using propofol and remifentanil. A nasal airway was inserted in the patients undergoing the MAC procedure, and oxygen was supplied via nasal prongs. During anesthesia, the patients were monitored for end-tidal carbon dioxide level, tidal volume, respiratory rate, oxygen saturation, electrocardiogram, non-invasive blood pressure, body temperature, and heart rate.
Data in this study were retrospectively obtained from the electronic medical records of the Samsung Medical Center. We collected data including patient demographics (age, sex, past medical history, and body mass index), tumor characteristics (tumor location, endoscopic tumor morphology, gross and pathologic tumor size, tumor specimen size, and tumor histopathology), and procedure-related factors (procedure time, anesthetic time, sedation method, and adverse events). The details of the pathologic findings, such as depth of invasion, lateral and vertical margins of the tumor, lymphatic and vascular involvement, and the presence of ulceration, were obtained for EGC cases.
The study’s primary outcomes were the frequencies of en bloc resection and complete resection. The secondary outcomes were the associated adverse events (bleeding and perforation). En bloc resection was defined as a single resection. Complete resection was defined as en bloc tumor resection with histologically confirmed lateral and vertical tumor-free resection margins. Curative resection, defined as complete resection without submucosal invasion of 500 μm or deeper, lymphatic invasion, or vascular involvement, was assessed in EGC cases. We also compared the procedural duration, defined as the time from the first observation of the lesions to complete lesion removal, including hemostasis, for the two anesthetic method groups. Perforation was defined as evidence of free air in the radiographic findings after the ESD procedure. ESD-related bleeding was defined as the need for hemostatic procedures after ESD.
We performed a propensity score (PS)-matched (1:1) analysis to compare patients who underwent GA with those who underwent MAC. The PS matching was based on the pathology of the tumor diagnosed before ESD, endoscopic tumor size, number of lesions, and the location of the tumor.
The baseline characteristics are summarized as mean ± - standard deviation (SD) or as frequencies (percentages). Categorical variables were compared using the chi-squared test or Fisher’s exact test, and continuous variables were compared using the Student’s t-test. Logistic regression was performed, and the odds ratio was calculated to identify factors associated with therapeutic outcomes and adverse events (p<0.05). The statistical analyses were performed using SPSS (version 25.0; IBM Co., Armonk, NY, USA) and R (version 3.6.1; R Foundation, Vienna, Austria).
The clinicopathologic characteristics of the cohort of patients with EGCs, adenomas, and NETs are shown in Supplementary Table 1, and those of the analyzed patients are shown in Table 1. The PS was used to match 94 patients who underwent GA with 94 patients who underwent MAC. Patients undergoing the two different anesthetic methods demonstrated similar clinicopathological characteristics.
Supplementary Table 2 shows the clinicopathological characteristics of the patients with EGC. After the PS matching before ESD based on histopathology, tumor size on endoscopy, number of lesions, and the location of the tumor, we performed a subgroup analysis of the patients with histologically confirmed EGC after ESD. Eight cases of adenoma were confirmed as EGC after ESD under GA, and nine cases of adenoma were confirmed as EGC in the MAC group. The subgroup analysis included 79 patients who underwent ESD under GA and 81 patients who underwent ESD under MA. Both anesthetic groups showed similar clinicopathological characteristics. The histological differentiation of EGCs was not significantly different in the two groups.
We compared the clinical outcomes of the two anesthesia groups of matched patients with EGCs, adenomas, and NETs. The duration of the procedure per lesion was longer in the GA group than in the MAC group (GA, 93.1±58.9 min; MAC, 74.7±42.8 min; p=0.015). Both anesthetic groups had high rates of en bloc (GA, 95.7%; MAC, 97.9%) and complete (GA, 81.9%; MAC, 84.0%) resection. Adverse events (perforation and bleeding) occurred in 15 patients (12 cases of perforation and 4 cases of bleeding) under GA and 8 patients (5 cases of perforation and 3 cases of bleeding) under MAC. One patient developed microperforation and bleeding after the procedure under GA. The statistical results showed no significant between-group differences (p=0.18) in the frequency of adverse events (Table 2).
The subgroup of patients with EGC showed similar results. The rates of en bloc (GA, 94.9%; MAC, 97.5%) and complete (GA, 79.7%; MAC, 81.5%) resections were high in both groups. However, the curative resection rates were lower than the complete resection rates due to the tumor characteristics. The curative resection rate in the GA group was significantly lower than that in the MAC group (GA, 58.2%; MAC, 74.1%; p=0.04) due to lymphatic invasion (GA, 26.6%; MAC, 9.9%; p=0.007) (Supplementary Table 3).
The multivariable analysis showed that the anesthetic method was not associated with complete ESD resection of gastric tumors (Table 3, Supplementary Table 4). A long resection margin was the only factor associated with complete resection of gastric tumors (Table 3). The regression analysis showed that the anesthetic method was not associated with the rate of adverse events (Table 4, Supplementary Table 5).
ESD is widely used to treat gastric tumors and often performed under anesthesia or traditional sedation administered by endoscopists [1,8,10]. Previous studies have compared the outcomes based on the sedatives or anesthetic methods used. However, there have been insufficient data on the comparison of the anesthetic methods (GA and MAC) used during difficult gastric ESD cases. To the best of our knowledge, this is the first study comparing the therapeutic outcomes and adverse events of ESD of tumors performed under GA and MAC in the upper region of the stomach.
In the present study, the en bloc resection rates of the total cohort comprising all types of gastric tumors were >95% for both anesthetic groups, and the complete resection rates were >80%; similar results were observed when the EGC cases were separately examined. In a previous meta-analysis of the clinical outcomes of ESD, the en bloc resection rate for 1,437 cases of EGC was 92.4%, and the complete resection rate for 1495 cases of EGC was 82.4%, regardless of the anesthetic method or tumor location [11]. Thus, the en bloc and complete resection rates in our study were above average. However, the rates of curative resection for EGC were relatively low (GA, 58.2%; MAC, 74.1%). A previous study on short-term post-ESD outcomes involving 712 patients in a prospective multicenter cohort study in Korea showed an en bloc resection rate of 97.3% and a curative resection rate of 86.8%. The authors suggested that non-curative resection was associated with large lesions, submucosal invasion, and moderately or poorly differentiated adenocarcinomas [12]. Furthermore, in that study, 26.1% of the patients had EGC lesions of sizes >2 cm; 33.1% of the lesions were moderately or poorly differentiated adenocarcinomas, and 16.0% of the lesions had invaded the submucosal layer [12]. In our study, 31.3% of EGCs were >2 cm in size, 68.8% were moderately or poorly differentiated adenocarcinomas, and 46.9% showed submucosal invasion. Thus, the lower curative resection rate in our study may have been due to the tumor characteristics of our study population.
In the present study, the number of cases of ESD-related perforation was 12 (12.8%) among patients receiving GA and five (8.5%) among those receiving MAC, and the respective rates of bleeding were 5.3% (four cases) and 4.5% (three cases). Among the EGC cases, perforation occurred in approximately ten patients (12.7%) who underwent GA and four patients (4.9%) who underwent MAC; the bleeding rates were 3.8% (three cases) and 3.7% (three cases), respectively. In a previous meta-analysis of ESD-related adverse events, a perforation rate of 4.3% was observed among 1,437 EGC cases, and bleeding occurred in 9.4% of 876 EGC cases, regardless of the anesthetic method or tumor location [11]. The higher incidence of perforations in our study may also be related to the tumor characteristics of our study population, as described. ESD involving the upper part of the stomach has a higher risk of adverse events such as perforation and bleeding due to the difficulty in positioning the ESD knife, the relatively thin gastric wall, and the associated vasculature; therefore, the procedure is prolonged and involves more advanced technical skills than procedures involving the lower portion of the stomach [4,13,14]. Moreover, a study reported that submucosal invasion of the EGC occurs more frequently in lesions located in the mid and upper parts of the stomach [5]. That study also showed that the rate of perforation was higher when the upper part of the stomach was involved (8% of 478 cases) than when the lower part was involved (0.5% of 478 cases) [15]. Another study showed that the risk of perforation for procedures involving the upper stomach was 4.9 times that for those involving other areas of the stomach after adjustment for submucosal invasion and dyslipidemia [16]. In the present study, perforation occurred in 9.0% of the analyzed patients. The perforation rates did not significantly differ in the two groups (p=0.18). However, the absolute number of perforation cases in the GA group was 2 times that of the MAC group in this study. All 17 cases of perforation in the analyzed patients had microperforation, which was treated by endoscopic clipping. No cases required surgical repair of the perforation. All patients were discharged after a short course of antibiotics without any further complications. Five patients underwent gastrectomy after the final pathologic reports of ESD specimens because of non-curative resection.
Our study hypothesized that the cases of ESD performed under GA would have better therapeutic outcomes and fewer adverse events than those not performed under GA because GA would prevent even subtle movements during the procedure. Results of previous studies involving esophageal or gastric ESD showed that GA decreases the risk of adverse events, compared with conventional sedation administered by endoscopists [2,17]. Furthermore, a previous study of esophageal ESD procedures conducted at our institute showed that the rate of en bloc resection was significantly higher, and the perforation rate was significantly lower in patients receiving GA than in those receiving traditional sedation. GA was shown to be associated with achieving complete resection and minimizing perforation [17]. Notwithstanding, there are only a few studies comparing GA and MAC, and MAC has been shown to provide more clinical benefits than GA. One retrospective study compared the endovascular angioplasty outcomes in patients with aortoiliac disease in the groups that received intra-operative GA or MAC. The rate of postoperative adverse events was significantly lower for procedures performed under MAC than for those performed under GA [18] Similarly, another retrospective study also suggested that MAC is a safe anesthetic method for mid-gestation pregnant women, and its use is associated with a lower rate of adverse events than GA [18].
Our study has limitations due to its retrospective design and the inclusion of procedures performed only at a single medical center. However, as a high-volume center that employs several technically advanced endoscopists, several gastric ESD cases have been performed and are available for inclusion. Since 2012, when ESD was introduced at our center, most procedures have been performed under traditional sedation or MAC. However, selection bias may exist because endoscopists require specific anesthetic methods before starting their procedures. Endoscopists tend to request GA for difficult cases, such as those involving tumors in the upper stomach or those that are presumed to be more invasive based on morphologic assessments. Moreover, procedures involving the mid-to-upper stomach are five times more likely to be performed under MAC than under GA. To overcome this limitation, we performed PS matching. There was also a limitation of statistical power in our study. We assume that this was due to the small number of analyzed patients and the consequent low statistical power. It was not possible to calculate an appropriate sample size because no previous data has been reported on GA and MAC. However, we believe that a large number of patients are required to demonstrate the difference with higher statistical power. Therefore, it may not be easy to conduct prospective studies. To overcome this limitation, a further study comparing the three groups, namely traditional sedation, MAC, and GA, and involving a larger sample is necessary. Although a cautious interpretation of the results on perforation is necessary, ESD can be safely and effectively performed using MAC in high-volume centers with specialized endoscopists.
In conclusion, our study results demonstrated good clinical outcomes of ESD of tumors in the mid-to-upper stomach, regardless of the anesthetic method used in our high-volume center. Furthermore, the results showed the non-inferiority of the safety and therapeutic outcomes of ESD procedures performed under MAC to those performed under GA. Considering that it is cost-effective and less invasive, gastric ESD under MAC may be superior to ESD under GA.
Supplementary Material
Supplementary Table 1.
Clinicopathologic Characteristics of Overall Study Patients
Supplementary Table 2.
Clinicopathologic Characteristics of Patients with Early Gastric Cancer
Supplementary Table 3.
Clinical Outcomes and Adverse Events in Patients with Early Gastric Cancer
Supplementary Table 4.
Factors Associated with Complete Resection in Patients with Early Gastric Cancer
Supplementary Table 5.
Factors Associated with Adverse Events in Early Gastric Cancer
Notes
Author Contributions
Conceptualization: Jae J Kim
Data curation: Jong-In Chang, Tae Jun Kim
Formal analysis: JIC, Na Young Hwang, Insuk Sohn
Funding acquisition: JJK
Investigation: TJK, JJK
Methodology: Yang Won Min, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Lee, JJK
Project administration: PLL, JJK
Resources: YWM, HL, BHM, JHL
Supervision: JJK
Validation: YWM, HL, BHM, JHL
Visualization: JIC, TJK
Writing-original draft: JIC, TJK
Writing-review&editing: JIC, TJK
REFERENCES
1. Yurtlu DA, Aslan F, Ayvat P, et al. Propofol-based sedation versus general anesthesia for endoscopic submucosal dissection. Medicine (Baltimore). 2016; 95:e3680.
2. Nonaka S, Kawaguchi Y, Oda I, et al. Safety and effectiveness of propofol-based monitored anesthesia care without intubation during endoscopic submucosal dissection for early gastric and esophageal cancers. Dig Endosc. 2015; 27:665–673.
3. Kim JH, Nam HS, Choi CW, et al. Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc. 2017; 31:1617–1626.
4. Nishiyama N, Kobara H, Fujihara S, et al. Endoscopic submucosal dissection for neoplasia of the greater curvature of the upper and middle stomach: j-shaped superficial cutting and splashed dissection. J Gastrointestin Liver Dis. 2019; 28:397–404.
5. Kang DH, Choi CW, Kim HW, et al. Location characteristics of early gastric cancer treated with endoscopic submucosal dissection. Surg Endosc. 2017; 31:4673–4679.
6. Kiriyama S, Gotoda T, Sano H, et al. Safe and effective sedation in endoscopic submucosal dissection for early gastric cancer: a randomized comparison between propofol continuous infusion and intermittent midazolam injection. J Gastroenterol. 2010; 45:831–837.
7. Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017; 15:194–206.
8. Park CH, Shin S, Lee SK, et al. Assessing the stability and safety of procedure during endoscopic submucosal dissection according to sedation methods: a randomized trial. PLoS One. 2015; 10:e0120529.
9. Yamaguchi D, Yamaguchi N, Takeuchi Y, et al. Comparison of sedation between the endoscopy room and operation room during endoscopic submucosal dissection for neoplasms in the upper gastrointestinal tract. BMC Gastroenterol. 2017; 17:127.
10. Yamashita K, Shiwaku H, Ohmiya T, et al. Efficacy and safety of endoscopic submucosal dissection under general anesthesia. World J Gastrointest Endosc. 2016; 8:466–471.
11. Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: a meta-analysis. World J Gastrointest Endosc. 2014; 6:555–563.
12. Choi IJ, Lee NR, Kim SG, et al. Short-term outcomes of endoscopic submucosal dissection in patients with early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2016; 10:739–748.
13. Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012; 26:2456–2464.
14. Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005; 17:54–58.
15. Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010; 45:30–36.
16. Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012; 27:907–912.
17. Song BG, Min YW, Cha RR, et al. Endoscopic submucosal dissection under general anesthesia for superficial esophageal squamous cell carcinoma is associated with better clinical outcomes. BMC Gastroenterol. 2018; 18:80.
18. Boulos NM, Burton BN, Carter D, Marmor RA, Gabriel RA. Monitored anesthesia care is associated with a decrease in morbidity after endovascular angioplasty in aortoiliac disease. J Cardiothorac Vasc Anesth. 2020; 34:2440–2445.
Fig. 1.
Flow diagram for the selection of the study patients. ESD, endoscopic submucosal dissection.
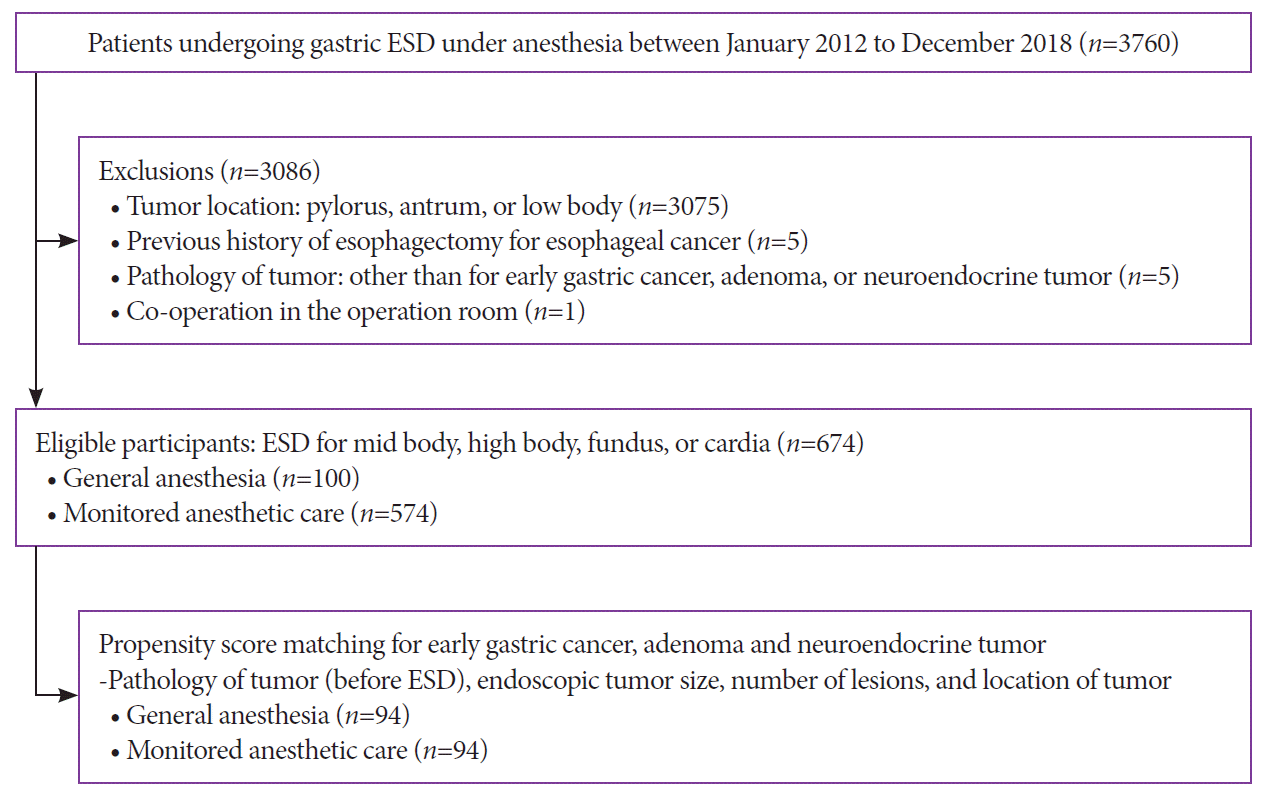
Fig. 2.
Endoscopic submucosal dissection procedure. (A) On the posterior wall of the high body, a 1.2-cm flat elevated mucosal lesion was noticed. (B) After the indigo carmine solution was sprayed for the visualization of the lesion, the lesion was marked with a needle knife. (C) Circumferential mucosal cutting was performed with a needle knife. (D) After submucosal injection of a mixture of normal saline, epinephrine, and indigo carmine, the submucosal layer was dissected with an IT2 knife. (E) A procedure-induced artificial ulcer was observed. (F) The resected specimen was fixed on a board for pathologic examination.
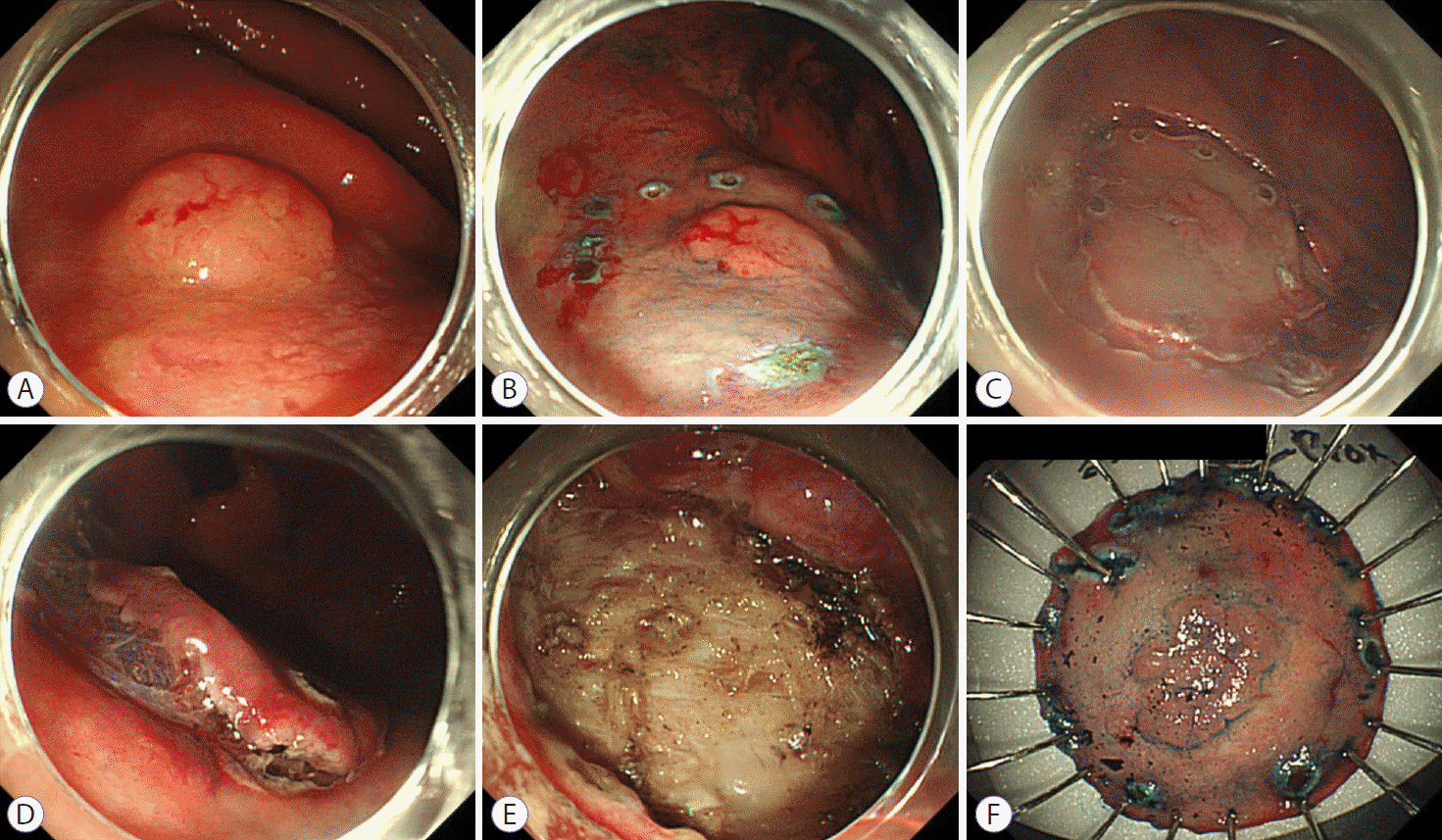
Table 1.
Clinicopathologic Characteristics of Patients with Early Gastric Cancer, Adenoma, and Neuroendocrine Tumor
Table 2.
Clinical Outcomes and Adverse Events of Patients with Early Gastric Cancer, Adenoma, and Neuroendocrine Tumor
Table 3.
Factors associated with Complete Resection in Patients with Early Gastric Cancer, Adenoma, and Neuroendocrine Tumor
Table 4.
Factors Associated with Adverse Events in Patients with Early Gastric Cancer, Adenoma, and Neuroendocrine Tumor




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download