CASE REPORT
A 26-year-old male patient with no particular medical history visited Asan Medical Center for the evaluation of intermittent bloody stools that he had been complaining of for the past 5 years. The patient denied any abdominal pain, weight loss, or change in bowel habits. The patient had no specific family history, especially of colorectal cancer or hereditary diseases. Initial vital signs were normal. Blood pressure of 110/70 mmHg, pulse rate of 70/min, respiration rate of 18/min, and body temperature of 36.8°C were recorded. Physical examination revealed normal bowel sounds sound with no tenderness or palpable mass in the abdomen. There were no cutaneous café-au-lait spots, axillary freckling, or plexiform neurofibroma. Initial colonoscopy revealed multiple sessile polyps of varying diameters, dispersed between the descending and sigmoid colon. The mucosal surface of the polyps was intact, with no erosion or ulcer (
Fig. 1). Histopathological examination of the biopsied tissue showed scattered ganglion cells admixed with a proliferation of spindle cells in the expanded lamina propria of the mucosa. Immunohistochemical staining showed ganglion cells with positive neuronal nuclei (NeuN), spindle cells with positive S-100 protein, and neuronal components with positive synaptophysin (
Fig. 2). These findings were consistent with the diagnosis of colonic ganglioneuroma. Laboratory tests were performed to investigate the presence of coexisting hereditary diseases. Serum calcitonin was 1.9 pg/mL (normal range, 0–10 pg/mL) and carcinoembryonic antigen was 2.2 ng/mL (normal range 0–6). Qualitative test for vanillylmandelic acid was negative. Computed tomography scan of the abdomen and pelvis, thyroid ultrasound, and esophagogastroduodenoscopy were performed to exclude MEN 2B and Cowden syndrome, which did not show any specific findings. Magnetic resonance imaging of the brain showed no brain or optic nerve tumors. The patient was diagnosed with colonic diffuse ganglioneuromatosis without any hereditary diseases.
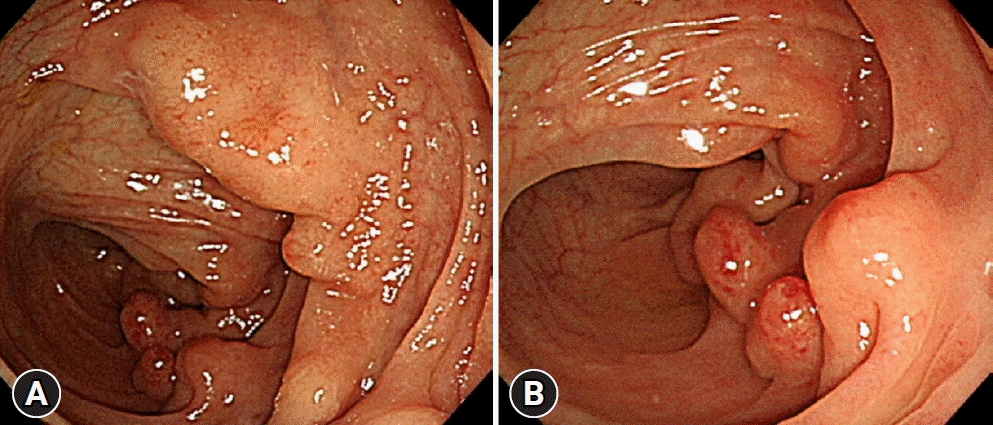 | Fig. 1.Initial colonoscopic findings of colonic diffuse ganglioneuromatosis. (A) Colonoscopy revealed the presence of multiple sessile polyps of varying diameters in the descending colon. (B) Some sessile polyps showed a hyperemic surface, but the overlying mucosa was intact with no definitive erosion or ulcer. 
|
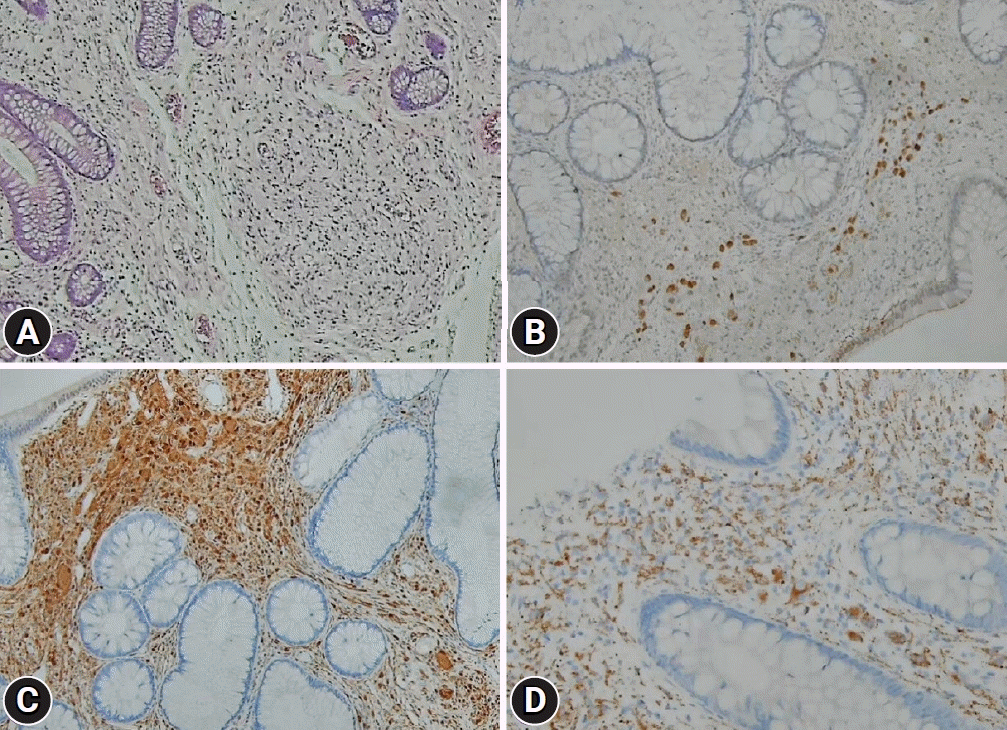 | Fig. 2.Microscopic and immunohistochemical findings. (A) Histological examination of the biopsied tissue showed proliferation of the ganglion and spindle cells in the lamina propria (hematoxylin & eosin, ×100). Immunohistochemical stainings showed diffuse positivity for (B) neuronal nuclei (×200), (C) S-100 protein (×200), and (D) synaptophysin (×400). 
|
The patient was followed up with regular surveillance colonoscopy at an interval of 1 to 2 years. Surveillance colonoscopy, 12 years after the initial diagnosis, revealed a 2.9 cm subpedunculated polyp in the sigmoid colon. The mucosal surface was hyperemic with type IV and V
I Kudo pit patterns (
Fig. 3). Mucosal biopsy revealed adenocarcinoma. The patient underwent total colectomy. Histologic examination revealed moderately differentiated adenocarcinoma confined to the mucosa arising from a tubular adenoma in the sigmoid colon, and diffuse ganglioneuromatosis with involvement of the entire colonic wall from the descending to the sigmoid colon (
Fig. 4). Pathological tumor staging was pTis, N0, and M0 (American Joint Committee on Cancer staging system, 8th edition). The patient was healthy without recurrence on follow-up 7 months after surgery.
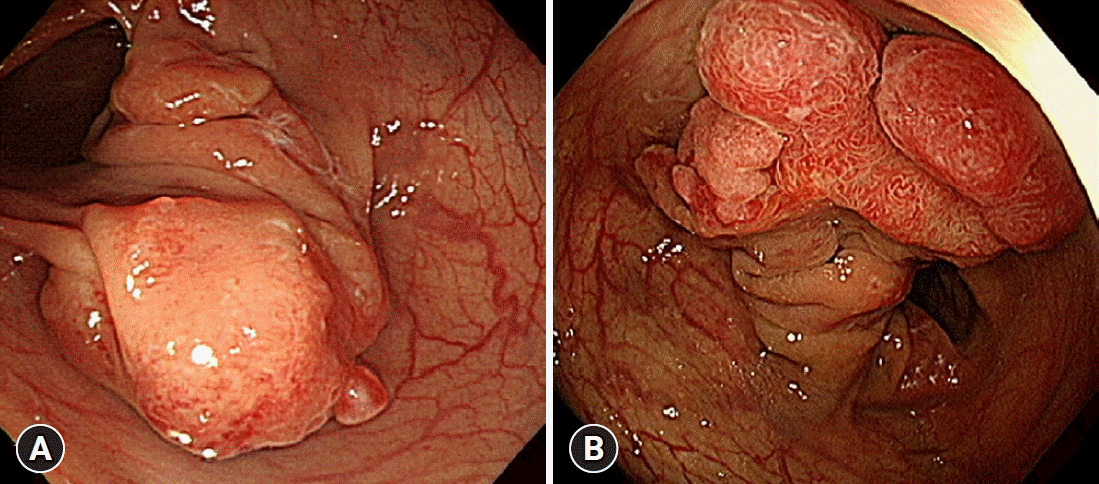 | Fig. 3.Colonoscopic findings of colon cancer in the background of colonic diffuse ganglioneuromatosis. (A) Initial colonoscopy at the time of diagnosis of colonic diffuse ganglioneuromatosis showed a large mass with intact, smooth overlying mucosa in the sigmoid colon. A very thick stalk-like structure was noted at the base of the mass. (B) Surveillance colonoscopy, 12 years after the initial diagnosis of colonic diffuse ganglioneuromatosis, showed a 2.9 cm mass at the same location as the initial lesion. The overlying mucosa was hyperemic with type IV and VI Kudo pit patterns. The stalk became more prominent. 
|
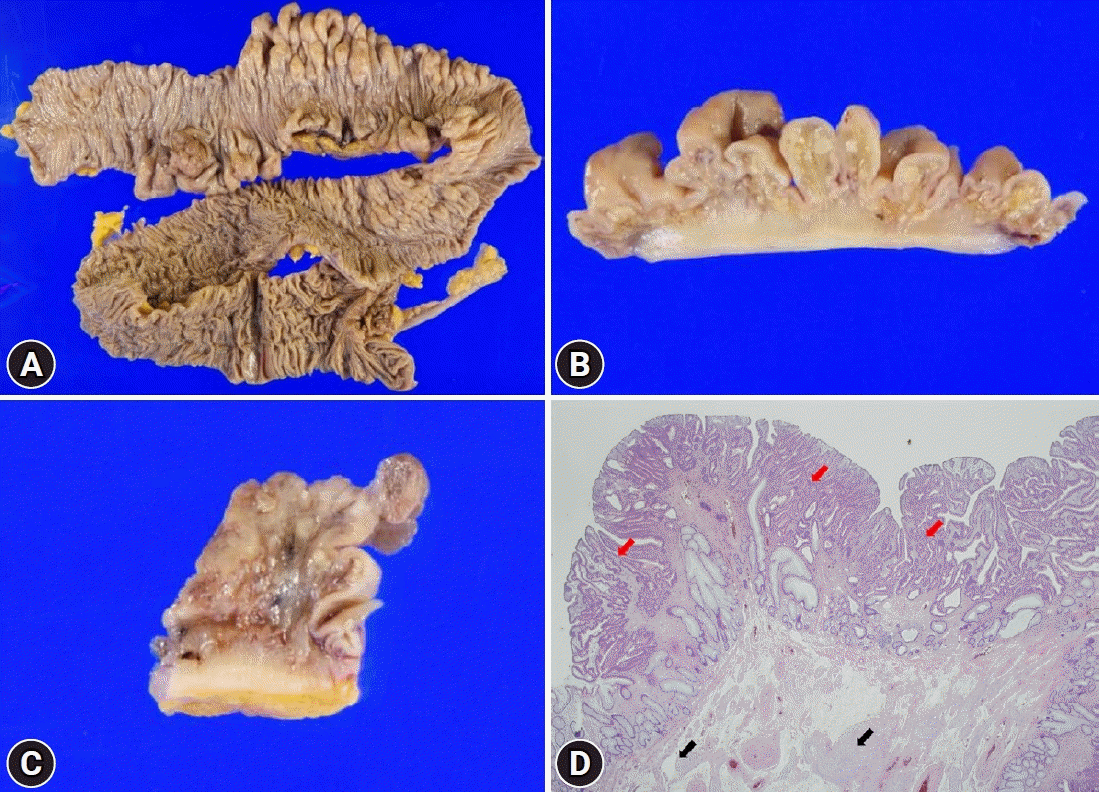 | Fig. 4.The surgical specimen after total colectomy. (A) Multiple polypoid lesions with colonic wall thickening from the descending to the sigmoid colon were observed. (B) A closer view showed diffuse ganglioneuromatosis with involvement of the entire colonic wall. (C) The subpedunculated lesion in the sigmoid colon showed adenocarcinoma arising from tubular adenoma. (D) Microscopic findings showed ganglion cell clusters (black arrow) in the area adjacent to moderately differentiated adenocarcinoma (red arrow; hematoxylin & eosin, ×40). 
|
Go to :

DISCUSSION
Ganglioneuroma, which originates from the neural crest, is considered a benign tumor composed of ganglion cells, supporting cells, and nerve fibers.
1 Although ganglioneuroma can occur in any part of the body in which autonomic nervous system is present, it develops most commonly in the adrenal medulla, posterior mediastinum, and retroperitoneum, where major sympathetic ganglia are present. It is the most common tumor of the sympathetic nervous system in adults.
2 Gastrointestinal ganglioneuroma is very rare, and colorectum is the location where ganglioneuroma occurs most commonly out of the entire gastrointestinal tract. It may occur either alone or in association with various hereditary diseases such as MEN 2B, NF type 1, familial adenomatous polyposis, juvenile polyposis, tuberous sclerosis, and Cowden syndrome.
3,
4
Ganglioneuromas of the gastrointestinal tract can be divided into three groups: (1) polypoid ganglioneuroma, (2) ganglioneuromatous polyposis, and (3) diffuse ganglioneuromatosis.
4 Polypoid ganglioneuroma is the most common form of gastrointestinal ganglioneuroma. It comprises of a single or a small number of polyps confined to the mucosa or submucosa. It grossly resembles a hyperplastic polyp, a juvenile polyp, or an adenoma.
4 It is not related to systemic diseases and has a good prognosis since it is a benign tumor. Ganglioneuromatous polyposis is defined as multiple, often more than 20, sessile or pedunculated ganglioneuromatous polyps. Diffuse ganglioneuromatosis is characterized by poorly demarcated nodular and disseminated lesions. It is composed of diffuse intramural or transmural proliferation of ganglioneuromatous tissues that involve the enteric plexus. These lesions can produce stricture-like thickening of the bowel wall or distort the surrounding tissue architecture.
4,
5 Diffuse ganglioneuromatosis can exist solitarily in the gastrointestinal tract or as a component of a systemic disease associated with MEN 2B or NF type 1.
Symptoms of gastrointestinal ganglioneuroma can vary from asymptomatic to abdominal pain, constipation, chronic diarrhea, bowel obstruction, and gastrointestinal bleeding, depending on the anatomical location and size of the tumor.
Ganglioneuroma is histologically diagnosed by identifying ganglion cells in the tissue. In general, hematoxylin and eosin staining is sufficient for histological diagnosis. However, immunohistochemical staining can be helpful in confirming the diagnosis by identification of ganglion cells using NeuN, neuron-specific enolase, synaptophysin, neurofilament protein, and vasoactive intestinal peptide. In addition, neural tissue composed of spindle cells can be identified using S-100 protein, vimentin, and glial fibrillary acidic protein.
4 Ganglioneuromas should be differentiated from neurofibromas and schwannomas. Since there are no ganglion cells in a neurofibroma or schwannoma, these differential diagnoses can be ruled out by confirming the presence of ganglion cells in the tissue.
6
There is no established association between gastrointestinal ganglioneuromas and malignant tumors. Polypoid ganglioneuroma has been reported to have a good prognosis with no malignant potential.
7 However, some studies have reported that ganglioneuromatous polyposis and diffuse ganglioneuromatosis might be accompanied by adenomatous polyps or colorectal cancer, although the incidence is rare. The first case of cecal adenocarcinoma surrounded by diffuse ganglioneuromatosis in a 54-year-old male patient was reported by Snover et al.
8 in 1981. Other reports described patients presenting with coexisting hereditary diseases, colonic ganglioneuroma, and colorectal neoplasia. Tomita et al.
9 reported a case of a 42-year-old male patient diagnosed with NF type 1 and diffuse ganglioneuromatosis with well-differentiated adenocarcinoma in the rectum that was treated with ileocolectomy and chemotherapy. Trufant et al.
10 reported another case of a 42-year-old male patient diagnosed with Cowden syndrome and gangliomatous polyposis with colonic adenomatous polyps and signet-ring adenocarcinoma in the colon with lymph node metastasis. Kanter et al.
11 suggested that ganglioneuromatous polyposis might be a premalignant condition in their report of a 40-year-old male patient with ganglioneuromatous polyposis and coexisting colorectal cancer in the same lesion.
In our case, the patient was initially diagnosed with colonic diffuse ganglioneuromatosis without any hereditary diseases. He was followed up with regular annual or biennial surveillance colonoscopy for 12 years, when colorectal cancer was diagnosed. Because genetic and epigenetic studies on the surgically resected colon cancer specimens were not performed in our case, we could not determine whether the colon cancer caused by diffuse ganglioneuromatosis developed through the general carcinogenic pathway, such as the adenoma-carcinoma sequence or the serrated pathway. Investigation of the carcinogenic pathways may help determine the etiopathogenesis of colon cancer caused by colonic ganglioneuroma, which should be performed in future studies.
Our case raises several issues. First, we suggest that colonic diffuse ganglioneuromatosis can be associated with colorectal cancer, similar to several previous reports showing the coexistence of ganglioneuroma and colorectal neoplasia.
8,
9 In our case, colon cancer was diagnosed as a ganglioneuromatous mass lesion (
Fig. 3), which suggests the possibility of the premalignant nature of ganglioneuroma. However, the suggestion that ganglioneuroma may itself directly develop into colorectal cancer remains controversial because ganglioneuroma is a tumor originating from the submucosal layer whereas colorectal adenocarcinoma develops from the epithelial layer. Therefore, we suggest that diffuse ganglioneuromatosis of the colorectum may lead to chronic mucosal inflammation that may result in colorectal cancer, such as colitic cancer caused by chronic inflammatory bowel diseases. Further studies, including histological and genetic investigations, are needed to confirm the possible association between ganglioneuroma and colorectal cancer and its carcinogenic mechanism. The second point raised by our case is the importance of regular surveillance colonoscopy for colonic diffuse ganglioneuromatosis. The stage of colorectal cancer in our patient was pTis, N0, and M0, which is a very early stage, contrary to the advanced stages necessitating chemotherapy mentioned in previous reports.
9,
10 We believe that the detection of carcinoma at this early stage can be attributed to the regular surveillance colonoscopy. Although, currently, there is no established consensus on the necessity of surveillance colonoscopy and its appropriate interval for early detection of adenocarcinoma in the background of gastrointestinal ganglioneuromatosis, we suggest a 1 to 2-year interval in surveillance colonoscopy based on our experience and recommendations in the literature.
In summary, we presented a case of diffuse ganglioneuromatosis that was accompanied by colon cancer diagnosed at an early stage by regular surveillance colonoscopy. Colonic ganglioneuroma, especially ganglioneuromatous polyposis and diffuse ganglioneuromatosis, may be associated with colorectal cancer, and regular surveillance colonoscopy should be considered for this condition.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download