Abstract
Acute liver failure due to malignant melanoma is uncommon. We presents a case of acute liver failure secondary to hepatic infiltration of a malignant melanoma. An 86-year-old man was admitted with elevated liver enzymes and an increased lactate dehydrogenase level. His condition progressed to acute liver failure, but the etiology of liver failure was unclear. Esophagogastroduodenoscopy was performed to evaluate dyspepsia, which showed signs indicative of malignant melanoma. Based on the endoscopy findings and elevated liver enzyme levels, liver biopsy was performed to confirm the presence of malignant melanoma. Hepatic infiltration of malignant melanoma was observed histologically. However, massive and diffuse liver metastasis is very rare and difficult to identify on imaging studies. If the etiology of liver failure is unclear, diffuse metastatic melanoma infiltration should be considered as differential diagnosis. Early liver biopsy can help to clarify the diagnosis.
Melanoma is an aggressive malignancy of melanocytes, which are pigment-producing cells that originate from the neural crest and migrate to the skin, meninges, mucous membranes, and eyes [1]. More than 97% of all melanomas are diagnosed by a known primary site, most often involving the skin. Melanoma is rarely diagnosed without an obvious primary site, and such rare cases are referred to as melanoma of unknown primary [2-3].
Malignant melanoma is an aggressive malignancy that tends to metastasize beyond its primary site. The most common metastatic sites are the regional lymph nodes, liver, lungs, bones, and brain. The prognosis is better for patients with skin and subcutaneous metastases than for those with metastases to the liver, bone, or brain [1]. Liver metastases are diagnosed in 10-20% of patients with melanoma, but significant hepatic dysfunction is uncommon [4]. Only few cases of spontaneous hepatic rupture, severe hepatitis, and acute liver failure due to malignant melanoma infiltration have been reported. Particularly, acute liver failure due to malignant melanoma has rarely been reported worldwide. Here, we present a case of acute liver failure secondary to hepatic infiltration of a malignant melanoma.
An 86-year-old man was admitted to the emergency room for complaints of nausea, vomiting, and abdominal pain in the previous week. He had a history of hypertension and dementia, but his vital signs were normal. The abdomen was soft and non-distended with tenderness in the right upper quadrant and left upper quadrant.
The laboratory data after admission showed abnormal liver function tests that raised the suspicion of acute hepatitis: total bilirubin, 1.54 mg/dL (normal, 0.1–1.2 mg/dL), aspartate aminotransferase 184 U/L (normal, 19–48 U/L), alanine aminotransferase 149 U/L (normal, 13–40 U/L), alkaline phosphatase 245 IU/L (normal, 40–130 IU/L), γ-glutamyl transferase 235 IU/L (normal, 4–63 IU/L). The serum lactate dehydrogenase level rose to 1,780 U/L (normal, 135–225 U/L), platelet count had dropped to 116,000/µL (normal, 150,000–450,000/μL), and prothrombin time rose to 15.2 seconds (normal, 10–13 seconds). Viral serological and autoimmune markers were negative. Computed tomography showed no evidence of focal lesions in the liver (Fig. 1). Ultrasound showed edematous wall thickening of the gallbladder, which was suspected to be secondary to acute hepatitis.
The patient denied the use of alcohol or any toxic drugs, including herbs. Diagnosis of acute hepatitis of unknown etiology was made, and appropriate treatment with hepatotonics was commenced. Five days after admission, the patient had persistent nausea and dyspepsia. Esophagogastroduodenoscopy revealed multiple black pigmented spots and nodules on the entire stomach, and a round, deep ulcer with black pigmented base at the lesser curvature of the antrum (Fig. 2). Malignant melanoma was suspected, and forceps biopsy was performed. Magnetic resonance imaging showed multiple high signal intensity nodules without definite focal mass-like lesions in the liver on T2-haste, T2-weighted, and diffusion-weighted sequences (Fig. 3).
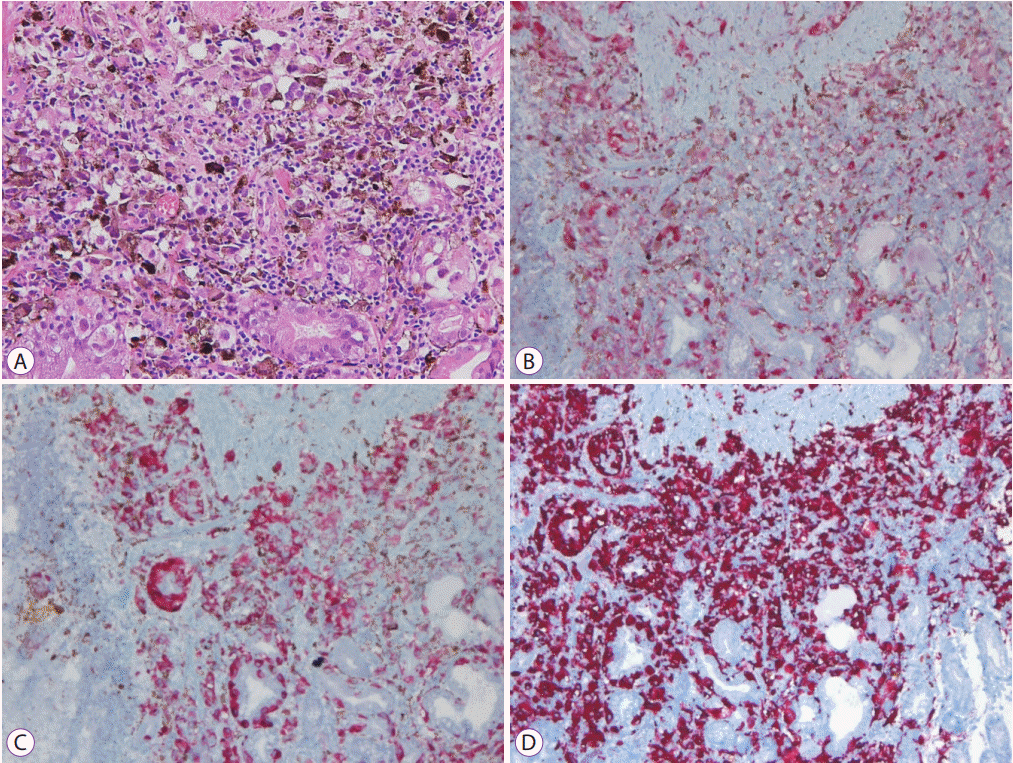
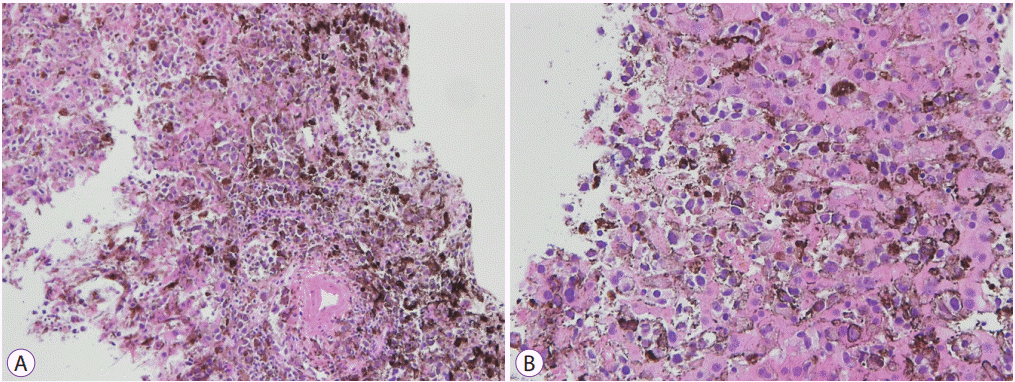
Seven days after admission, there was no decrease in the aspartate aminotransferase or alanine aminotransferase levels despite treatment. Moreover, the levels of total bilirubin and other liver enzymes increased prominently. A percutaneous liver biopsy was performed to clarify the cause of the elevated liver enzymes. An interventional radiologist technically preferred a percutaneous liver biopsy rather than a transjugular liver biopsy. After the biopsy, the patient’s condition rapidly deteriorated with worsening liver function tests. The serum liver function test values increased continuously and peaked at 2,104 U/L for aspartate aminotransferase, 416 U/L for alanine aminotransferase, and 7.60 mg/dL for total bilirubin. The platelet count was 27,000/µL, and prothrombin time was 33.9 seconds. His mental status changed from alert to deep drowsiness. The patient developed metabolic acidosis with multiple organ failure and passed away one day later. The biopsy results were reported post-mortem. Histological examinations of the biopsy specimens of the stomach, duodenum, and liver showed extensive amounts of nucleoli with distinct large nuclei and infiltrating tumor cells with brown pigmentation (Figs. 4 and 5). Normal liver parenchyma and sinusoids were not present at a discernible level. Immunohistochemistry for S-100 and HMB45 antibodies is relatively sensitive, and the antibodies are specific markers for melanoma [5]. The markers for S-100, HMB45, and melan-A were positive in the patient’s biopsy report.
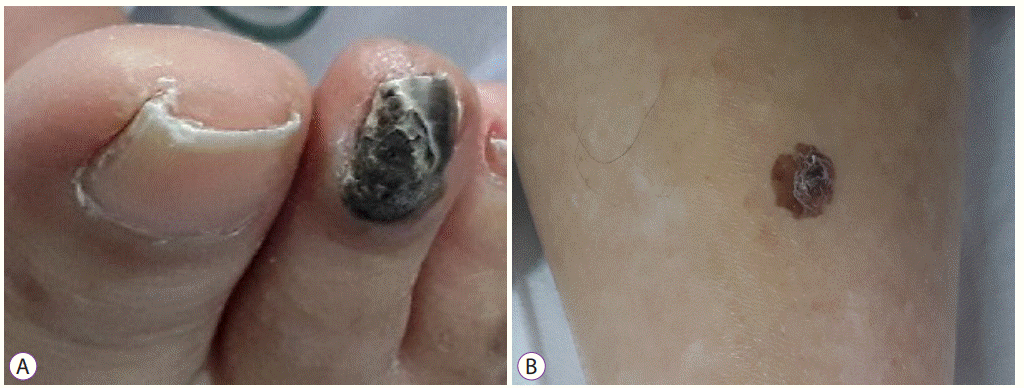
Physical examination of the patient’s body showed several skin lesions suggestive of melanoma. The right second toenail color changed to black beyond the nail fold, and a brown spot with an irregular border and shape was found on the lower leg (Fig. 6). However, as the patient’s condition worsened, the guardian did not consent for skin biopsy. Thus, we could not perform the workup of the primary site of the malignant melanoma, nor was the diagnosis of uveal melanoma excluded.
Acute liver failure secondary to hepatic infiltration of a malignant melanoma is uncommon. Some of the reported cases showed rapid progression of liver failure and systemic organ failure and subsequent death. Most of them were identified in the post-mortem studies, as in vivo biopsy is very difficult to perform due to severe coagulopathy present during acute liver failure [6].
The signs and symptoms of hepatic infiltration of malignant melanoma are difficult to distinguish from the primary hepatic causes. Hepatic infiltration of malignant melanoma has been associated with mild to moderately elevated serum aminotransferases, alkaline phosphatase, and bilirubin levels. Abnormal levels of lactate dehydrogenase have also been associated with tumor involvement in the liver. Majority of the patients show nodular infiltration in the liver, but massive diffuse liver metastasis is very rare and difficult to identify on imaging studies [6]. Histologically, melanoma dissemination is characterized by leukemoid tumor infiltration of the liver sinusoids leading to sinusoidal obstruction, hepatocellular death, and fulminant liver failure [7]. Due to these histological features in melanoma dissemination, the liver may appear normal on imaging studies. It seems that diffuse leukemoid infiltration of malignant melanoma within the sinusoids does not cause actual lesions in the liver parenchyma.
Our patient presented with dyspepsia, and esophagogastroduodenoscopy findings were strongly suggestive of disseminated melanoma. Hence, we suspected that the elevated liver enzymes were associated with melanoma, and a liver biopsy was performed to confirm this diagnosis. The hepatic sinusoids were diffusely infiltrated by the malignant melanoma cells, and the hepatocytes were extensively replaced by malignant melanoma cells at the time of biopsy. Therefore, no focal lesions were observed in the liver. After the liver biopsy, the patient’s clinical condition rapidly deteriorated to acute liver failure. He developed multiple organ failure and passed away even before the workup of the primary site of malignant melanoma could be performed.
In summary, this was a report of an unusual case of acute liver failure associated with metastatic melanoma that replaced the vast majority of the liver tissue. If the etiology of liver failure is unclear, diffuse metastatic melanoma infiltration can be considered as a differential diagnosis. It is difficult to identify the etiology using laboratory tests and imaging studies, and liver biopsy is difficult to perform due to severe coagulopathy present during acute liver failure. Nevertheless, early liver biopsy would be helpful to clarify the diagnosis.
REFERENCES
1. Curti BD, Leachman S, Urba WJ. Cancer of the Skin. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s principles of internal medicine 20th ed. New York (NY): McGraw-Hill Education; 2018. p. 522-530.
2. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998; 83:1664–1678.
3. Dasgupta T, Bowden L, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obste. 1963; 117:341–345.
4. Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001; 136:950–955.
5. Te HS, Schiano TD, Kahaleh M, et al. Fulminant hepatic failure secondary to malignant melanoma: case report and review of the literature. Am J Gastroenterol. 1999; 94:262–266.
6. Montero JL, Muntané J, de las Heras S, Ortega R, Fraga E, De la Mata M. Acute liver failure caused by diffuse hepatic melanoma infiltration. J Hepatol. 2002; 37:540–541.
7. Schlevogt B, Rehkämper J, Hild B, Schmidt HH. Hepatobiliary and pancreatic: fulminant liver failure from diffuse leukemoid hepatic infiltration of melanoma. J Gastroenterol Hepatol. 2017; 32:1795.
Fig. 1.
Computed tomography scan shows mild fatty change and small amount of ascites without focal lesions in the liver.
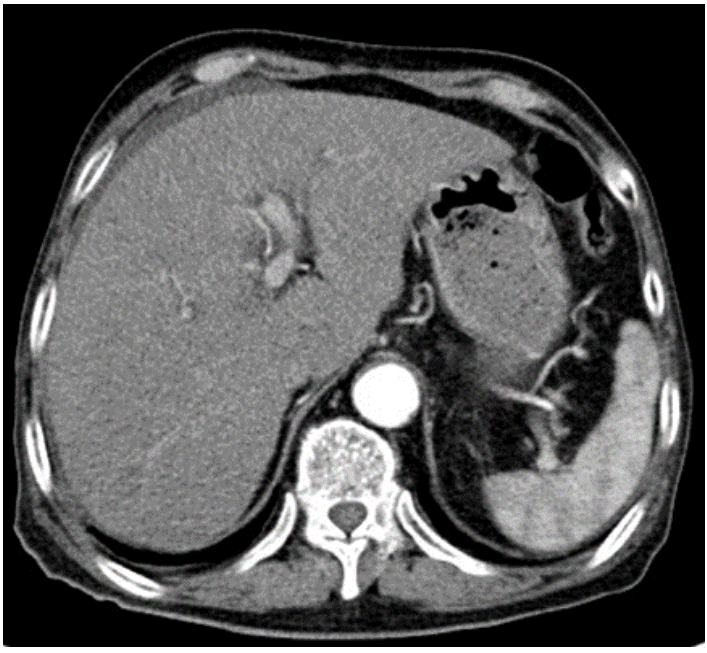
Fig. 2.
Endoscopic findings. (A), (B) Multiple black pigmented spots and nodules are seen on the entire stomach. (C) A round deep ulcer with black pigmented base is seen at the lesser curvature of the antrum. (D) Multiple shallow ulcers with black pigmented base are seen in the duodenum.
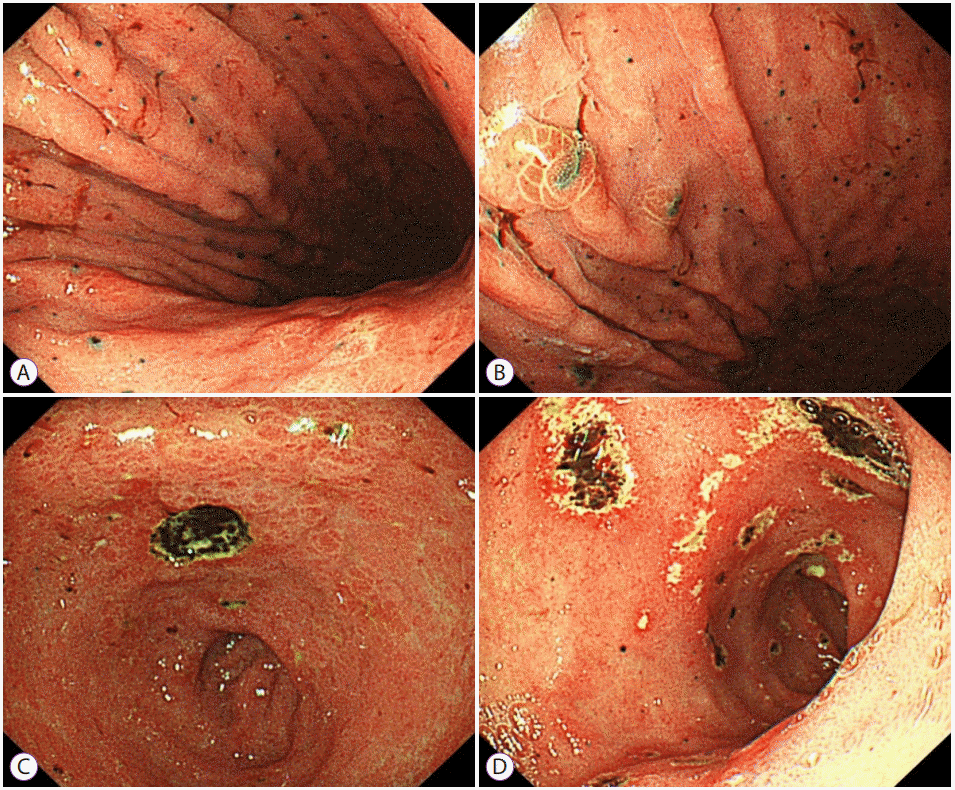
Fig. 3.
Magnetic resonance imaging shows multiple high signal intensity nodules without defined focal mass-like lesions in the liver on T2 blade.
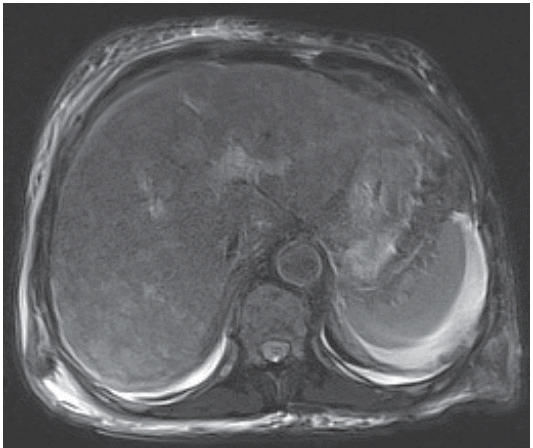
Fig. 4.
Stomach biopsy findings. (A) Stomach biopsy findings show nucleoli with distinct large nucleus and infiltrating tumor cells with brown pigmentation (hematoxylin & eosin stain, ×200). (B, C, D) Immunohistochemically, the tumor cells are positive for S-100, HMB45, and melan-A (immuhohistochemical stain, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download