Abstract
We reviewed 7 patients with unsuccessful endoscopic hemostasis using covered self-expandable metal stent (CSEMS) placement for post-endoscopic sphincterotomy (ES) bleeding. ES with a medium incision was performed in 6 and with a large incision in 1 patient. All but 1 of them (86%) showed delayed bleeding, warranting second endoscopic therapies followed by CSEMS placement 1–5 days after the initial ES. Subsequent CSEMS placement did not achieve complete hemostasis in any of the patients. Lateral-side incision lines (3 or 9 o’clock) had more frequent bleeding points (71%) than oral-side incision lines (11–12 o’clock; 29%). Additional endoscopic hemostatic procedures with hemostatic forceps, hypertonic saline epinephrine, or hemoclip achieved excellent hemostasis, resulting in complete hemostasis in all patients. These experiences provide an alert: CSEMS placement is not an ultimate treatment for post-ES bleeding, despite its effectiveness. The lateral-side of the incision line, as well as the oral-most side, should be carefully examined for bleeding points, even after the CSEMS placement.
Endoscopic sphincterotomy (ES) is an established procedure in endoscopic retrograde cholangiopancreatography (ERCP) for the management of common bile duct (CBD) stones and treatment for biliary diseases. Post-ES bleeding is its most important adverse event (AE), with a frequency of 1%–48%, including a considerable rate of overall mortality (0.1%–0.3%) [1-4]. Although endoscopic interventions, such as balloon tamponade, conventional injection therapy, coagulation with sphincterotomes or hemostatic forceps, and hemoclip application, are generally performed first, traditional hemostasis still does not achieve perfect resolution. Over the years, the use of covered self-expandable metal stents (CSEMSs) has been reported as a useful hemostatic technique for massive and refractory post-ES bleeding as the powerful self-expanding force of the stent could compress the bleeding point [5-8]. This effectual technique might be considered as an ultimate technique to address this AE. However, a small but significantly minor exception was encountered. Previous studies showing the use of CSEMS placement for post-ES bleeding reported rare cases of unsuccessful hemostasis with CSEMS, but reported them as exceptional cases in the literature. The characteristics of these patients have not yet been fully investigated. The present case series retrospectively analyzed patients with unsuccessful CSEMS placement to control post-ES bleeding in order to clarify the clinical features of unsuccessful hemostasis with CSEMS for post-ES bleeding.
Since 2014, CSEMS placement has been performed following unsuccessful attempts at hemostasis using conventional endoscopic procedures at Nagoya City University Hospital and Aichi Medical University Hospital when post-ES bleeding requiring endoscopic intervention occurs.
Using a side-viewing video duodenoscope (JF-260V or TJF-260V; Olympus, Tokyo, Japan), standard wire-guided sphincterotomes (Clevercut; Olympus, or Correctome; Boston Scientific, Marlborough, MA, USA) and a generator with an automatic cutout system (Endo-cut mode, ICC200; Erbe, Tübingen, Germany) were used for ES, making an incision in the 11–12 o’clock direction. When bleeding occurred following ES, endoscopic combination therapies were performed to achieve hemostasis with either coagulation with sphincterotomes, balloon tamponade, hypertonic saline epinephrine (HSE) injection, hemoclip application, or hemostatic forceps, as conventional endoscopic hemostasis. When continuous bleeding persisted even after these hemostatic attempts, a CSEMS should be placed across the major papilla. Seven patients with unsuccessful endoscopic hemostasis using CSEMS placement were retrospectively identified from 2014 to 2020.
This study was approved by the institutional review board at Nagoya City University Hospital and Aichi Medical University Hospital and conducted in accordance with the Declaration of Helsinki.
Representative images of patients are shown in Figure 1, and patients’ clinical characteristics are shown in Table 1. Patients comprised 4 men and 3 women, ranging in age from 49 to 80 years. Five patients underwent ES for the extraction of CBD stones, and 2 (with chronic pancreatitis and hilar cholangiocarcinoma) underwent ES for biliary drainage. Comorbidities were chronic renal failure (CRF) requiring hemodialysis in 3 patients, liver cirrhosis in 2, cerebral infarction requiring aspirin treatment in 1, and atrial fibrillation treated with warfarin in 1. One patient had CRF treated with warfarin and clopidogrel. With regard to incision, ES with a medium incision (extending from one- to two-thirds of the total length of the ampulla) was performed in 6 patients and with a large incision (extending to the superior margin of the intramural bile duct) in 1.
In the first ERCP with initial ES, all patients showed immediate bleeding after ES, but subsequent endoscopic conventional hemostasis was achieved in all but 1 patient, in whom combinational hemostasis using coagulation with sphincterotome, balloon tamponade, and HSE injection was attempted, but failed. These 6 patients showed delayed bleeding requiring second endoscopic therapies (coagulation with sphincterotome and balloon tamponade), followed by CSEMS placement at 1–5 (median, 2) days after the initial endoscopic hemostasis. The median procedure time of the hemostatic attempt before the CSEMS placement was 18 (range, 8–42) min. For CSEMS placement, a WallFlex Biliary RX Stent (Boston Scientific) with a size of 10×60 mm was deployed in all patients. Bleeding was still uncontrolled even after the CSEMS placement in all but 1 patient. In this one patient, temporary hemostasis seemed to be achieved by CSEMS placement at the time of placement, but resulted in unsuccessful hemostasis and required additional emergent endoscopy on the subsequent day. The bleeding point was at the 3 o’clock position in 3 patients, and at 9 and 11–12 o’clock in each of the 2 patients, respectively. Additional endoscopic hemostatic procedures with hemostatic forceps (n=3), HSE (n=3), or hemoclip (n=1) achieved effective hemostasis, resulting in the achievement of complete hemostasis in all patients. Bleeding severity was graded according to the lexicon of the American Society for Gastrointestinal Endoscopy [9]. Based on this grading, 6 patients were categorized as “moderate” (defined as bleeding requiring transfusion, admission to an intensive care unit for 1 night, interventional radiology, or prolonged hospitalization for 4–10 nights), and 1 patient was “mild” (defined as bleeding that did not match to moderate or severe). With regard to AEs, 1 patient developed pancreatitis after the CSEMS placement. All patients underwent subsequent stent removal without complications. The median duration of stent placement was 17 (range, 0–80) days.
Post-ES bleeding is a significant, well-recognized AE of ERCP procedures. Although effective hemostasis by conventional endoscopic therapies can achieve control of this AE in majority of patients, these therapies could not resolve obstinate bleeding in a certain proportion of patients. CSEMS placement is now recognized as a useful method to overcome these issues [5-8]. However, as shown in this case series, a small but significant minor uncontrolled bleeding with CSEMS placement is encountered in practical experience. To the best of our knowledge, this study presents the first report on incomplete hemostasis with CSEMS for post-ES bleeding in a relatively large case series. As a matter of fact, incomplete compression of the bleeding point must be a conceivable refractory factor. Focusing on this point, factors related to refractory bleeding and their characteristics are summarized in Table 2. All patients showed some comorbidity resulting in a predilection for bleeding. In majority of patients, a medium incision was performed (6/7; 86%). All of them had an incision made in the right direction (11–12 o’clock). All patients displayed bleeding immediately after ES. However, six of them (86%) could temporarily control bleeding by conventional hemostasis on initial ES. These results indicated that unsuccessful hemostasis with CSEMS was more frequent in the CSEMS placement for delayed bleeding than in the CSEMS placement for immediate bleeding on initial ES.
Iwasaki et al. inferred in their article that CSEMS may achieve insufficient compression of the bleeding point at the oral-most side in patients with a large ES incision [10]. In fact, 1 patient with persistent bleeding originating at the 11–12 o’clock position indeed had a large ES incision. The large ES incision created a space between the CSEMS and the cut edge, which caused insufficient compression of the oral-most side. However, other patients had a medium ES incision. Moreover, the lateral side (3 or 9 o’clock) (5/7; 71%) was a more frequent bleeding point than the oral-most side (11–12 o’clock) (2/7; 29%) (Fig. 2). These results indicated that CSEMS compression in the lateral direction was insufficient even with a medium ES incision. The lateral area after ES consists of a bare papillary wall and the cut edge of the bile duct. With the medium ES incision, the cut edge of the bile duct could be fully compressed by CSEMS, but the bare papillary wall might not be fully compressed.
Although poor visibility due to continuous bleeding and the clot obscured detection of the bleeding point, CSEMS placement, even if unsuccessful in achieving hemostasis, helped clarify the bleeding point and was successfully followed by complete hemostasis with hemostatic forceps, HSE, or hemoclip in all patients. In this respect, transient tamponade of the ES incision site with endoscopic papillary balloon dilation using a dilation balloon catheter is also a good endoscopic procedure candidate that can play a similar role.
Another impressive characteristic was that the nature of bleeding in these patients seemed relatively mild on initial ES, without spurting, supported by the following evidence: (1) all scheduled endoscopic procedures (extraction of CBD stones and biliary drainage) were achieved in all patients in advance of CSEMS placement; and (2) first ERCP with initial ES did not require CSEMS placement for hemostasis in 6 patients.
In conclusion, we encountered 7 patients with unsuccessful hemostasis from CSEMS placement for the treatment of postES bleeding. This case series could provide an alert regarding CSEMS placement as follows: (1) CSEMS placement is not the ultimate option and requires attention even after the stent placement, especially regarding delayed ES bleeding in patients with comorbidities; (2) careful identification of the bleeding point around the lateral side of the papilla as well as the oral-most side is necessary when complete hemostasis is not achieved after the CSEMS placement; and (3) hemostatic forceps, HSE, and hemoclip are effective because the bleeding point to arrest hemorrhage became relatively apparent after the CSEMS compression.
Notes
Author Contributions
Conceptualization: Michihiro Yoshida, Tadahisa Inoue, Itaru Naitoh
Data curation: MY, TI, IN, Kazuki Hayashi, Yasuki Hori, Makoto Natsume, Naoki Atsuta, Hiromi Kataoka
Formal analysis: MY, YH, MN
Methodology: MY, TI, IN, KH
Project administration: KH
Supervision: HK
Writing-original draft: MY
Writing-review&editing: IN
REFERENCES
1. Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996; 335:909–918.
2. Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998; 48:1–10.
3. Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001; 96:417–423.
4. ASGE Standards of Practice Committee, Chandrasekhara V, Khashab MA, et al. Adverse events associated with ERCP. Gastrointest Endosc. 2017; 85:32–47.
5. Itoi T, Yasuda I, Doi S, Mukai T, Kurihara T, Sofuni A. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011; 43:369–372.
6. Canena J, Liberato M, Horta D, Romão C, Coutinho A. Short-term stenting using fully covered self-expandable metal stents for treatment of refractory biliary leaks, postsphincterotomy bleeding, and perforations. Surg Endosc. 2013; 27:313–324.
7. Cochrane J, Schlepp G. Comparing endoscopic intervention against fully covered self-expanding metal stent placement for post-endoscopic sphincterotomy bleed (CEASE Study). Endosc Int Open. 2016; 4:E1261–E1264.
8. Inoue T, Ibusuki M, Kitano R, et al. Early covered self-expandable metal stent placement is effective for massive post-endoscopic sphincterotomy bleeding. Dig Dis Sci. 2020; 65:3324–3331.
9. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
10. Iwasaki E, Itoi T, Kanai T. Metal stent for refractory post-ES bleeding: is this the ultimate treatment modality? Endosc Int Open. 2016; 4:E1265–E1266.
Fig. 1.
Endoscopic images from case 1. (A) An endoscopic image of the duodenal papilla before endoscopic sphincterotomy (ES). (B) On initial ES, post-ES bleeding was temporarily controlled after the conventional endoscopic hemostasis. (C) Emergency endoscopy showed delayed bleeding from the duodenal papilla 1 day after the initial ES. Balloon tamponade is attempted for hemostasis, resulting in failure, followed by placement of a covered self-expandable metal stent (CSEMS). (D) Bleeding continued from the 9 o’clock position, because of insufficient compression by the CSEMS. (E) Complete hemostasis by hemostatic forceps.
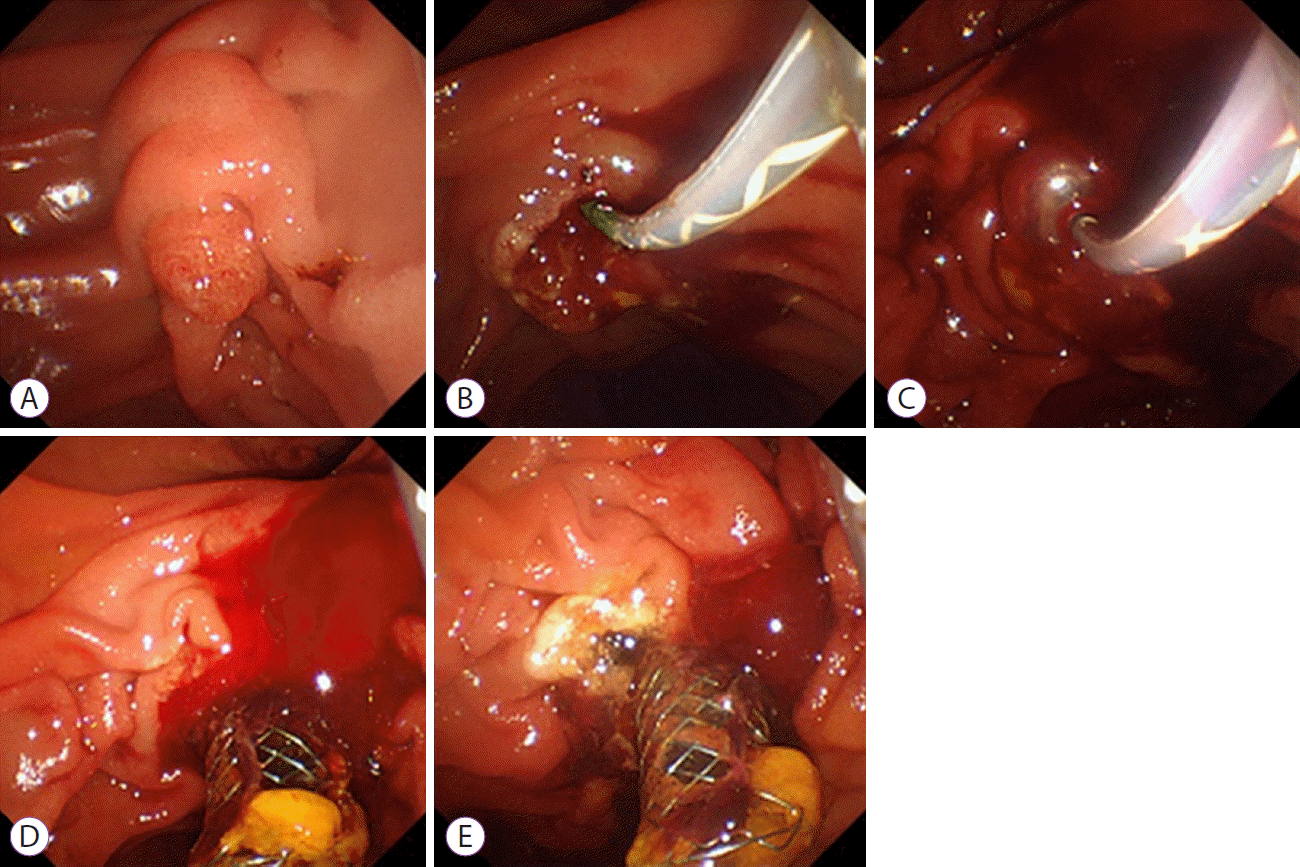
Fig. 2.
Schematic characteristics of unsuccessful hemostasis using covered self-expandable metal stent placement for post-endoscopic sphincterotomy bleeding. The bleeding point was in the 3 o’clock position in 3 cases (43%), and 9 and 11–12 o’clock in 2 cases, respectively (29%). Hemostatic forceps (n=3), hypertonic saline epinephrine (HSE) (n=3), and hemoclip (n=1) achieved complete hemostasis.
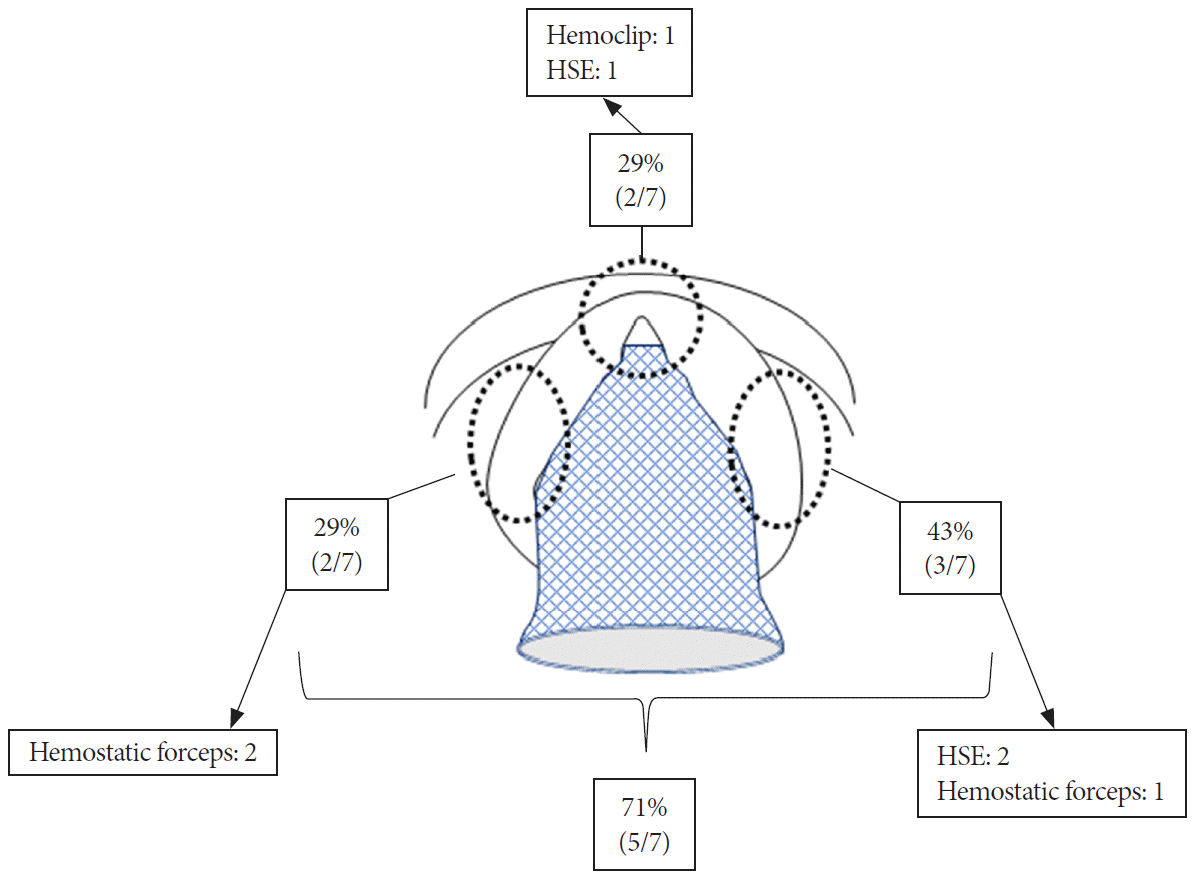
Table 1.
Patient Characteristics, Treatment, and Outcomes of Endoscopic Hemostasis for Post-Endoscopic Sphincterotomy Bleeding
Table 2.
CSEMS, covered self-expandable metal stent; ES, endoscopic sphincterotomy; HSE, hypertonic saline epinephrine.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download