Abstract
The market for hearing technology is evolving—with the emergence of hearables, it now extends beyond hearing aids and includes any ear-level devices with wireless connectivity (i.e., wireless earbuds). However, will this evolving marketplace bring forth opportunities or challenges to individuals’ hearing health care and the profession of audiology and otolaryngology? The debate has been ongoing. This study explores the wide spectrum of hearables available in the market and discusses the necessity of high-quality clinical evidence prior to the implementation of over-the-counter devices into clinical practice.
Hearing loss is a major health issue that affects various aspects of life (e.g., communication, academic performance, and social activities) [1,2]. With population aging, the number of individuals with hearing loss is expected to reach one in 10 individuals; thus, early and active interventions are in high demand [1-3]. A variety of hearing devices, such as hearing aids (HAs), middle ear implants, bone-anchored HAs, and cochlear implants (CIs), are typically prescribed in current clinical practice to manage hearing loss [4-6]. However, barriers to hearing devices still exist, leading to a low uptake rate. These barriers include price, maintenance cost, discomfort, and stigma associated with hearing devices [7]. The MarkeTrak V study undertaken by Kochkin [7] reported in 2000 that poor benefits, background noise, price and cost, and sound quality were reasons for the nonuse of HAs. In an effort to address these issues, alongside substantial improvements in technology, “hearables” have been emerging in the market [8-11].
Hunn [11] first coined the term “hearables” in 2014 to refer to any device that is capable of wireless connection. The definition has now expanded to any ear-level device that has wireless connectivity [9], including wireless headphones and earphones as well as smart HAs. The history of hearables goes back to 1855, when an early version of a stethoscope was developed. This category of products then evolved into tele-operator headsets, portable headphones, noise-canceling headphones, earbuds, Bluetooth headsets, and finally the current hearables available in the market [10]. The emergence of hearables has greatly expanded the horizons for hearing loss management as they are cheaper and more accessible than traditional HAs [12,13].
The arrival of Bragi led to dramatic growth in hearables in 2014, with 50 million dollars of crowdfunding, and the evolution of hearables further sped up with the introduction of AirPods, AirPods Pro, and Galaxy Buds, which had better audio quality than previous options and wireless charging [14]. Hunn [14] recently estimated that around 630 million hearables would be available in 2025, with a total market size of $80 billion. The market size for earbuds is anticipated to continue growing, as well as that of HAs, especially with more user acceptance of these devices. Headphones, in contrast, are expected to show slower growth [14].
This paper reviews current hearables in the market and the need for sustained efforts to continue examining these products before dispensing them in clinics as alternative amplification devices for individuals with hearing loss.
HAs are sound amplifying devices that generally consist of a microphone, speaker, amplifier, and battery [15]. The microphone picks up sounds, which are then amplified by the amplifier and sent to the speaker [15]. HAs are currently prescribed as the first option for hearing loss management. After the introduction of digital signal processing in 1996, hearing technology has greatly advanced [16]. The advances include HA accessories, noise reduction, wireless connectivity, and directional microphones [17,18]. Instead of disposable batteries, rechargeable batteries are now available for most HAs [19]. In addition, modern HAs can utilize artificial intelligence technology to improve audibility and quality of life [20]. Numerous studies have reported that HAs improve communication, academic performance, and quality of life [21-25]. For example, Cox et al. [26] recruited 25 people with mild and moderate sensorineural hearing loss and compared the effectiveness of premium HAs with that of basic HAs through speech testing, questionnaires, and diaries. Speech performance improved when participants wore the four HAs (two premium and two basic HAs). Participants’ diaries revealed positive feedback regarding the devices. However, a statistically significant difference was not observed between the premium and basic HAs. Tognola et al. [27] investigated the impact of age, cognition, and hearing loss on HA benefits in older adults (≥65 years). Pure-tone audiometry, aided threshold testing, speech reception testing in quiet and noise, the Montreal Cognitive Assessment, and questionnaires (the International Outcome Inventory of Hearing Aids, the Hearing Handicap Inventory for Elderly-Screening, and the Abbreviated Profile of Hearing Aid Benefit) were performed. Statistically significant improvement was observed with aided thresholds. In their responses to questionnaires, participants reported better satisfaction and less difficulty in communication when wearing HAs [27]. Direct associations of questionnaire outcomes with hearing loss and the aided threshold and an indirect association of questionnaire outcomes with cognitive test performance were observed through multivariate, correlational, and regression analyses [27]. Most et al. [25] assessed the benefits of unilateral and bilateral HAs in 80 HA users using the Speech, Spatial, and Qualities questionnaire. Although better speech and spatial performance (e.g., speech in quiet and localization) was observed with bilateral HAs, no statistical significance was observed on the qualities scale. Scientific evidence shows that two HAs are better than one, but it is important to consider personal preferences as well. For example, Cox et al. [28] recruited 98 participants and conducted a 12-week field trial with HAs in three conditions (left, right, and both ears). At the final visit, the participants were asked to report their wearing preference and the results showed that 43 out of 94 participants preferred to use one HA. Snapp [22] discussed the benefit of contralateral routing of signal (CROS) technology for individuals with single-sided deafness. Conventional CROS HAs transfer sounds picked up on the poor-hearing side to the better-hearing (or normal-hearing) side. The author reported that while CROS technology does not restore binaural hearing, it allows individuals with single-sided deafness to hear better on their poor-hearing side by improving the signal-to-noise ratio; they can be more aware of sound and hear speech better in noise. In addition, advances in technology and design led to increased acceptance and adoption of CROS technology [22,29].
In addition to the well-documented benefits of HAs, it is important to mention that HAs have technical specifications based on the guidelines established by the American National Standards Institute (ANSI) and the International Electrotechnical Commission (IEC) [30]. The ANSI and IEC standards provide references for HA functionality; therefore, quality control is systematically performed for these devices, allowing agreement throughout the industry on a regulatory as well as professional level [31,32]. For example, the Food and Drug Administration (FDA) can evaluate a product based on these guidelines and decide whether or not the product can be sold in the market. On a professional level, audiologists and HA dispensers perform electroacoustic testing and verify the functionality of HAs before patients are fitted with the devices.
First introduced in the late 1970s [33], bone conduction devices are mainly recommended for people with conductive and mixed hearing loss and single-sided deafness [34,35]. Unlike conventional HAs, bone conduction devices deliver sounds directly to the cochlea through bone vibration [34]. Typically, there are two types of bone conduction devices: percutaneous and transcutaneous. Percutaneous devices consist of an abutment, an osseointegrated screw, and an external processor, and involve skin penetration [36,37]. Transcutaneous devices, in contrast, have two types: passive and active. While passive transcutaneous bone conduction devices have a titanium implant, a magnet, and an external device, active transcutaneous bone conduction devices consist of an implant transducer and an external device, and the two components are connected via magnetic coils [37]. Along with HAs, bone conduction devices are also known to improve speech discrimination and sound localization [38]. Priwin et al. [38] performed threshold testing in the sound field, sound localization testing, and speech testing in quiet and noise in 12 adults who wore bilateral bone-anchored HAs. The results revealed improvement in sound field thresholds, sound localization, and speech recognition when the participants wore bone-anchored HAs on both sides. Similar benefits have been noted in children [39]. den Besten et al. [39] reported that bone conductive devices provided benefits in sound localization and lateralization in children wearing percutaneous bone conduction devices. When comparing unilateral and bilateral benefits, children performed better when they were wearing the devices in both ears.
In individuals with unilateral hearing loss, it has been suggested that sounds from the poor-hearing side can have a negative influence on sound localization as there is a mismatch between sounds picked up on the poor- and better-hearing sides [34]. Active research on this topic is necessary since mixed findings have been reported. For instance, while Monini et al. [35] reported improvements in sound localization with the use of conventional bone conduction devices for individuals with single-sided deafness, the experiments conducted by Agterberg et al. [34] showed no impact of conventional bone conduction devices on sound localization for people with single-sided deafness—they neither improved nor interfered with sound localization.
CIs, in general, are considered for severe hearing loss that is difficult to manage with conventional HAs [40,41]. A CI is composed of an external (speech processor) and an internal (electrode array) component [42]. Similar to HAs, the external component transfers sound from the environment to the internal component, which receives and processes the sound. From the surgical technique to aesthetics, CIs have also undergone significant advances [43,44]. The early models of CIs had only one channel, but research into CIs has led to the development of multichannel devices. CIs have a different number of electrodes and features depending on the manufacturer. In 2008, a hybrid model combining a CI and HA was launched and different types of CIs (off-the-ear and behind-the-ear) have been introduced [43]. CI companies are currently working with HA manufacturers to provide communication benefits to individuals with hearing loss. There is abundant scientific evidence regarding the effectiveness of CIs in aural rehabilitation [45-53]. In 2009, Laske et al. [47] assessed the subjective and objective benefits of CIs in adults. The sentence test in quiet and noise, sound localization test, and a questionnaire were carried out in bilateral and unilateral conditions. The results showed better speech understanding in quiet and noisy environments in the bilateral condition. In terms of sound localization, although statistically not significant, participants showed better performance in the bilateral condition for the summation and squelch effects. However, statistical significance was observed with the head shadow effect when the sound was presented on the CI side. Questionnaire results were also better for the bilateral condition. Moon et al. [49] examined the correlations between speech performance and various factors associated with CIs (e.g., age of deafness onset). Speech performance was evaluated using mono- and bi-syllable and sentence tests. The authors reported no correlation between age of deafness onset and speech performance, but a significant correlation was found between speech performance and the percentage of the patient’s life with moderate-to-profound hearing loss before CI placement, indicating that the duration of hearing loss before implantation may predict speech performance after implantation [49]. A study involving unilateral and bilateral CI users showed improvements in speech recognition, health-related quality of life, and tinnitus distress [45].
Direct-to-consumer hearing devices refer to hearing devices that can be purchased without a healthcare professional [13]. In other words, an individual can purchase this type of device online or at retail shops [13]. Direct-to-consumer devices come in different types: headset amplifiers, television amplifiers, ear-level neckband personal sound amplification products (PSAPs), earlevel wireless PSAPs, and combinations of a smartphone, amplification application, and wired earbuds [54]. For example, Sound World Solutions CS50+ and Tweak Focus+T are behind-the-ear devices, while Able Planet Ps2500amp is an in-ear device. Jabees BHearing is a neckband-type PSAP. Direct-to-consumer hearing devices have various functions. In some, users can only adjust volume and for others, users can select a mode (e.g., café) and even “program” the devices with mobile devices [55]. Major corporations have already entered the market. Samsung Electronics released a wearable augmented reality device, Galaxy Buds Pro (wireless earbuds), in January 2021. This wearable augmented reality device utilizes a smartphone and earbuds for a customized listening experience (Fig. 1) [12]. Samsung Galaxy Buds Pro use the Galaxy Wearable application and users can benefit from features such as active noise canceling and ambient sound.
Direct-to-consumer hearing devices gained traction when the President’s Council of Advisors on Science and Technology advocated for the use of PSAPs and over-the-counter (OTC) devices in addition to HAs for those with mild and moderate hearing loss to address the increasingly serious issue of hearing loss due to aging in 2015 [56]. The National Academies of Sciences, Engineering, and Medicine also reported that the FDA needed to create a new category regarding OTC devices for individuals with mild to moderate hearing loss [57]. Regulations regarding this new category were supposed to be proposed by August 2020, but the process was delayed due to the coronavirus disease 2019 pandemic [58,59]. Recently, in July 2021, an executive order was signed by President Joe Biden regarding OTC HAs, calling for the Health and Human Services Administration to propose rules for OTC HAs within 120 days [60].
Considering the high unmet need (67%–86%) for hearing health care [57], direct-to-consumer devices gained attention as a possible option to overcome the current HA uptake barriers mentioned earlier. Firstly, direct-to-consumer devices are much cheaper than HAs. While it costs more than $2,300 to purchase an HA [61], the cost for direct-to-consumer devices ranges from $20 to $500 [62]. HAs tend to cost more than direct-to-consumer devices because professional services, such as device fitting and programming, are included in the price [63]. Secondly, direct-to-consumer devices do not require multiple visits to hearing health care professionals. HAs, in contrast, require multiple visits for professional services [63]. Bose Corporation just recently launched a product called “Bose Sound Control Hearing Aids” for $849.95. The price is still considerably lower than that of traditional HAs. However, it is important to note that the product is FDA-cleared, not FDA-approved. FDA clearance (510[k] clearance) indicates that the Bose product is safe and shows significantly equivalent performance when compared to devices already in the market in the US [64].
PSAPs and OTC devices are similar to HAs to some extent, but there is a clear regulatory distinction between the two. The main difference is the purpose of device use; HAs are for hearing loss compensation and PSAPs are for those without hearing loss. In addition, the United States FDA classifies air-conduction HAs as class I medical devices [55] and the delivery of HA-related services is regulated by state laws [57]. Currently, HAs need to be provided by licensed professionals, such as audiologists. In Korea, HAs are also defined by the Ministry of Food and Drug Safety as medical devices used to compensate for hearing loss. There are currently no regulations regarding PSAPs. This lack of regulation has contributed to significant variation in the quality and performance of the devices. To guide manufacturers and consumers in improving and selecting products, in 2017, the Consumer Technology Association collaborated with ANSI and released the “Personal Sound Amplification Performance Criteria (ANSI/CTA-2051)” [65]. The criteria used the ANSI and IEC standards for HAs (ANSI S3.22-2009, IEC-60118-0-2015, and IEC-60118-7-2005) as normative references. The criteria also provide three categories (categories 1, 2, and 3) for standardization, with category 1 being the highest level of performance specification. For example, category 1 includes frequency response bandwidth, frequency response smoothness, maximum acoustic output, input and output distortion control limits, and self-generated noise levels [65]. However, these criteria are only voluntary, meaning that manufactures are not obligated to follow ANSI/CTA-2051.
Research has actively investigated the potential of direct-to-consumer devices as a means to increase the accessibility and affordability of hearing healthcare [66-73]. Reed et al. [66] compared speech in noise performance between five PSAPs and a conventional HA and demonstrated that three of the five PSAPs showed similar improvements in speech understanding to that of the HA. Cho et al. [67] also examined performance of a PSAP, a basic HA, and a premium HA in individuals with mild, moderate, and moderate-to-severe sensorineural hearing loss. In the mild and moderate hearing loss group, the PSAP showed comparable performance to the HAs; no statistically significant result was observed between the three devices in terms of speech recognition. Most participants (41%) also preferred the PSAP over the basic (28%) and premium (31%) HAs. Seol et al. [68] undertook a similar study with a HA and PSAP pair, but a new type of hearable (a wearable augmented reality device) was included in the experiment. The electroacoustic characteristics of all devices met the four key tolerances (output sound pressure level, frequency range, equivalent input noise, and total harmonic distortion) set by the ANSI standards. Regarding speech perception, the findings were similar to those of previous studies to a certain extent—statistically significant improvements were observed for all devices for words, but not for sentences. The findings of the study demonstrated the potential of wearable augmented reality devices as an amplification alternative for those with mild and moderate hearing loss. However, the authors highlighted the significance of a close examination of device quality.
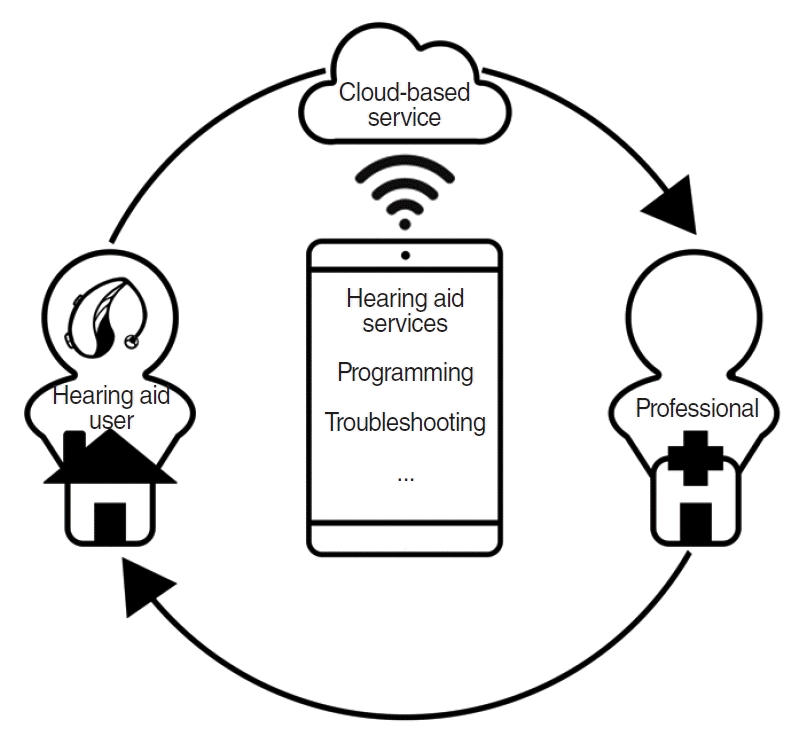
Smartphone applications have also been emerging in the hearable market due to their ubiquity. According to Manchaiah et al. [62], the average price range for smartphone applications is between $0 and $10. A representative example would be HA applications. Current HA applications not only provide volume and program adjustment, but also tinnitus management and remote assistance (Fig. 2). These HA applications allow users to customize their hearing profiles through the applications. There are also accessibility features built into smartphones. The iPhone has a “Live Listen” feature where the phone becomes a remote microphone and directly sends sounds to HAs when activated. For those using an iPhone and Made for iPhone HAs, a separate remote microphone might not be necessary. The “Live Listen” feature can be used for Apple’s audio devices (i.e., AirPods). For Android, Google partnered with GN Hearing and Cochlear to provide HAs with direct audio streaming with Bluetooth Low Energy. Google also has an application called “Sound Amplifier,” which amplifies ambient sound as well as sounds playing on the mobile device. Individuals can adjust the bass and treble and the amount of noise reduction. The “Hearing Enhancements” feature is also available in Samsung Galaxy smartphones, with options including “Hearing Aid Support,” “Amplify Ambient Sound,” “Adapt Sound,” “Left/Right Sound Balance,” and so on. Users can personalize their sound settings based on their age range and hearing test results, which they can complete on the phone through “Adapt Sound.” “Amplify Ambient Sound” is similar to Google’s “Sound Amplifier.” These applications currently require headphones and earphones—they do not work with HAs. Bluetoothcompatible HAs can be customized with “Hearing Aid Support.” When HAs are connected with the “Hearing Aid Compatibility” feature activated, HA volume can be adjusted. Aside from builtin smartphone accessibility features and HA applications, sound amplification applications are also available on Google Play and the Apple App Store. However, research on the effectiveness of these applications in managing hearing loss has been sparse [74,75]. In 2013, Amlani et al. [75] used a conventional HA and two smartphone applications to assess the utility of the applications. The electroacoustic characteristics of mobile devices and applications and individuals’ unaided and aided speech performance in noise were assessed and surveys were administered. Compared to the unaided condition, the use of the HA and applications significantly increased speech understanding in noise. Similar electroacoustic characteristics were observed between the HA, mobile device, and applications. De Sousa et al. [74] investigated the objective sound quality and subjective listening experience of four applications available on Google Play and the Apple App Store. In terms of objective sound quality, latency and the signal-to-noise ratio improvement were examined through an occluded ear simulator, Android smartphones, and an iPhone. The results showed variance in latency for all applications on the mobile devices. Furthermore, latency was significantly different between wired and wireless earbuds. Improvement in the signalto-noise ratio was observed, but variance was observed between the mobile devices. The subjective listening experience was examined using only one application that showed the best electroacoustic performance. Overall, most participants reported that the use of the application was beneficial for conversations in a quiet situation, but not in difficult listening situations.
From HAs to wearable augmented reality devices, the market for hearables now includes a wide variety of devices. Pre-existing studies have examined the quality of some hearables and suggested them as a gateway to hearing healthcare for individuals with mild and moderate hearing loss. Despite some evidence suggesting that hearables can be beneficial for people with mild to moderate hearing loss, it is important to note that research has only investigated a small sample of devices. Therefore, it is difficult to generalize the findings and further studies are necessary. The lack of regulations has led to variance in device quality, contributing to the mixed findings regarding device performance. The results of an electroacoustic analysis and simulated real-ear measurements for three basic and high-end PSAPs reported by Kim et al. [69] revealed that only some showed satisfactory performance. Two out of the three high-end devices met the electroacoustic tolerances and one basic and two high-end PSAPs provided an adequate amount of gain in simulated real-ear measurements. This means that depending on the quality of devices, some might not provide benefits for individuals with hearing impairment. In this respect, the need for high-quality clinical evidence, as well as regulations ensuring the safety and efficacy of these devices, is imperative in order to integrate hearables into clinical settings.
▪ The broad spectrum of hearables now includes not only hearing aids, but also any ear-level devices with wireless connectivity.
▪ Studies have shown the potential of hearables to be utilized as an alternative amplification option for individuals with mild and moderate hearing loss, but it is difficult to generalize the findings, as not all devices have been examined.
▪ This study reviews current hearables in the market and the need for high-quality clinical evidence regarding their quality and performance.
REFERENCES
1. National Research Council (US) Committee on Disability Determination for Individuals with Hearing Impairments. Hearing loss: determining eligibility for social security benefits. Washington (DC): National Academies Press;2004.
2. World Health Organization. Deafness and hearing loss [Internet]. Geneva: World Health Organization;2021 [cited 2021 Jul 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
3. World Health Organization. Addressing the rising prevalence of hearing loss. World Health Organization [Internet]. Geneva: World Health Organization;2018. [cited 2021 Jul 1]. Available from: https://apps.who.int/iris/handle/10665/260336.
4. Arlinger S. Negative consequences of uncorrected hearing loss: a review. Int J Audiol. 2003; Jul. 42 Suppl 2(23-24):2S17–20.
5. Solheim J, Kværner KJ, Falkenberg ES. Daily life consequences of hearing loss in the elderly. Disabil Rehabil. 2011; 33(23-24):2179–85.

6. Fortunato S, Forli F, Guglielmi V, De Corso E, Paludetti G, Berrettini S, et al. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol Ital. 2016; Jun. 36(3):155–66.
7. Kochkin S. MarkeTrak V: “Why my hearing aids are in the drawer”: the consumers’ perspective. Hear J. 2000; Feb. 53(2):34–6.
8. Johansen B, Flet-Berliac YP, Korzepa MJ, Sandholm P, Pontoppidan NH, Petersen MK, et al. Hearables in hearing care: discovering usage patterns through IoT devices. In : . Cham: Springer;2017. p. 39–49.
9. Hunn N. The market for hearable devices 2016-2020 [Internet]. London: WiFore Consulting;2016. [cited 2021 Jul 1]. Available from: http://www.nickhunn.com/wp-content/uploads/downloads/2016/11/The-Market-for-Hearable-Devices-2016-2020.pdf.
10. Plazak J, Kersten-Oertel M. A survey on the affordances of “hearables”. Inventions. 2018; Sep. 3(3):48.

11. Hunn N. Hearables: the new Wearables [Internet]. Nick Hunn;2014. [cited 2021 Jul 1]. Available from: https://www.nickhunn.com/hearables-the-new-wearables/.
12. Taylor B. Hearables: the morphing of hearing aids and consumer electronic devices. Audiol Today. 2015; 27(6):22–31.
13. Almufarrij I, Munro KJ, Dawes P, Stone MA, Dillon H. Direct-to-consumer hearing devices: capabilities, costs, and cosmetics. Trends Hear. 2019; Jan-Dec. 23:2331216519858301.

14. Hunn N. The hearables report 2020-2025 [Internet]. London: WiFore Consulting;2016. [cited 2021 Jul 1]. Available from: http://www.nickhunn.com/download/2457/.
15. Popelka GR, Gates GA. Hearing aid evaluation and fitting. Otolaryngol Clin North Am. 1991; Apr. 24(2):415–28.

17. Kerckhoff J, Listenberger J, Valente M. Advances in hearing aid technology. Contemp Issues Commun Sci Disord. 2008; Oct. 35(Fall):102–12.

18. ur Rehman MZ, Shah SI, Gilani SO, Jamil M, Amin F. An appraisal of the advancement of emerging technologies in hearing aids. Indian J Sci Technol. 2016; 9(25):1–6.
20. You E, Lin V, Mijovic T, Eskander A, Crowson MG. Artificial intelligence applications in otology: a state of the art review. Otolaryngol Head Neck Surg. 2020; Dec. 163(6):1123–33.

21. Choi AY, Shim HJ, Lee SH, Yoon SW, Joo EJ. Is cognitive function in adults with hearing impairment improved by the use of hearing AIDS. Clin Exp Otorhinolaryngol. 2011; Jun. 4(2):72–6.

22. Snapp H. Nonsurgical management of single-sided deafness: contralateral routing of signal. J Neurol Surg B Skull Base. 2019; Apr. 80(2):132–8.

23. Kwak SH, Kim D, Bae SH, Moon IS, Kim SH, Choi JY, et al. Effects of contralateral routing of signal hearing aids on audiological and academic performance in school-age children with unilateral hearing loss. Clin Exp Otorhinolaryngol. 2021; Aug. 14(3):355–8.

24. Mondelli MF, Souza PJ. Quality of life in elderly adults before and after hearing aid fitting. Braz J Otorhinolaryngol. 2012; Jun. 78(3):49–56.
25. Most T, Adi-Bensaid L, Shpak T, Sharkiya S, Luntz M. Everyday hearing functioning in unilateral versus bilateral hearing aid users. Am J Otolaryngol. 2012; Mar-Apr. 33(2):205–11.

26. Cox RM, Johnson JA, Xu J. Impact of advanced hearing aid technology on speech understanding for older listeners with mild to moderate, adult-onset, sensorineural hearing loss. Gerontology. 2014; 60(6):557–68.

27. Tognola G, Mainardi A, Vincenti V, Cuda D. Benefit of hearing aid use in the elderly: the impact of age, cognition and hearing impairment. Acta Otorhinolaryngol Ital. 2019; Dec. 39(6):409–18.

28. Cox RM, Schwartz KS, Noe CM, Alexander GC. Preference for one or two hearing AIDS among adult patients. Ear Hear. 2011; Mar-Apr. 32(2):181–97.

29. Finbow J, Bance M, Aiken S, Gulliver M, Verge J, Caissie R. A comparison between wireless CROS and bone-anchored hearing devices for single-sided deafness: a pilot study. Otol Neurotol. 2015; Jun. 36(5):819–25.
31. Ravn G, Preves D. Hearing aid-related standards and test systems. Semin Hear. 2015; Feb. 36(1):29–48.

32. Frye GJ. Understanding the ANSI standard as a tool for assessing hearing instrument functionality. Hear Rev. 2005; May. 12(5):22.
33. Hakansson B, Tjellstrom A, Rosenhall U, Carlsson P. The bone-anchored hearing aid: principal design and a psychoacoustical evaluation. Acta Otolaryngol. 1985; Sep-Oct. 100(3-4):229–39.

34. Agterberg MJ, Snik AF, Van de Goor RM, Hol MK, Van Opstal AJ. Sound-localization performance of patients with single-sided deafness is not improved when listening with a bone-conduction device. Hear Res. 2019; Feb. 372:62–8.

35. Monini S, Musy I, Filippi C, Atturo F, Barbara M. Bone conductive implants in single-sided deafness. Acta Otolaryngol. 2015; Apr. 135(4):381–8.

36. Hakansson B, Eeg-Olofsson M, Reinfeldt S, Stenfelt S, Granstrom G. Percutaneous versus transcutaneous bone conduction implant system: a feasibility study on a cadaver head. Otol Neurotol. 2008; Dec. 29(8):1132–9.
37. Ellsperman SE, Nairn EM, Stucken EZ. Review of bone conduction hearing devices. Audiol Res. 2021; May. 11(2):207–19.

38. Priwin C, Stenfelt S, Granstrom G, Tjellstrom A, Hakansson B. Bilateral bone-anchored hearing aids (BAHAs): an audiometric evaluation. Laryngoscope. 2004; Jan. 114(1):77–84.

39. den Besten CA, Vogt K, Bosman AJ, Snik AF, Hol MK, Agterberg MJ. The merits of bilateral application of bone-conduction devices in children with bilateral conductive hearing loss. Ear Hear. 2020; Sep/Oct. 41(5):1327–32.

40. Varadarajan VV, Sydlowski SA, Li MM, Anne S, Adunka OF. Evolving criteria for adult and pediatric cochlear implantation. Ear Nose Throat J. 2021; Jan. 100(1):31–7.

41. Sladen DP, Gifford RH, Haynes D, Kelsall D, Benson A, Lewis K, et al. Evaluation of a revised indication for determining adult cochlear implant candidacy. Laryngoscope. 2017; Oct. 127(10):2368–74.

42. Naples JG, Ruckenstein MJ. Cochlear implant. Otolaryngol Clin North Am. 2020; Feb. 53(1):87–102.

43. Hainarosie M, Zainea V, Hainarosie R. The evolution of cochlear implant technology and its clinical relevance. J Med Life. 2014; 7 Spec No. 2(Spec Iss 2):1–4.
44. Fouad YA. Advances in surgical and anesthetic techniques for cochlear implantation. In : Zanetti D, editor. Advances in rehabilitation of hearing loss. London: IntechOpen;2020.
45. Ketterer MC, Haussler SM, Hildenbrand T, Speck I, Peus D, Rosner B, et al. Binaural hearing rehabilitation improves speech perception, quality of life, tinnitus distress, and psychological comorbidities. Otol Neurotol. 2020; Jun. 41(5):e563–74.

46. Laszig R, Aschendorff A, Stecker M, Muller-Deile J, Maune S, Dillier N, et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol. 2004; Nov. 25(6):958–68.

47. Laske RD, Veraguth D, Dillier N, Binkert A, Holzmann D, Huber AM. Subjective and objective results after bilateral cochlear implantation in adults. Otol Neurotol. 2009; Apr. 30(3):313–8.

48. Kim E, Lee HJ, Kim HJ. Music perception ability of Korean adult cochlear implant listeners. Clin Exp Otorhinolaryngol. 2012; Apr. 5(Suppl 1):S53–8.

49. Moon IJ, Kim EY, Jeong JO, Chung WH, Cho YS, Hong SH. The influence of various factors on the performance of repetition tests in adults with cochlear implants. Eur Arch Otorhinolaryngol. 2012; Mar. 269(3):739–45.

50. Moon IJ, Kim EY, Park GY, Jang MS, Kim JH, Lee J, et al. The clinical significance of preoperative brain magnetic resonance imaging in pediatric cochlear implant recipients. Audiol Neurootol. 2012; Aug. 17(6):373–80.

51. Chang YS, Hong SH, Kim EY, Choi JE, Chung WH, Cho YS, et al. Benefit and predictive factors for speech perception outcomes in pediatric bilateral cochlear implant recipients. Braz J Otorhinolaryngol. 2019; Sep-Oct. 85(5):571–77.

52. Youm HY, Moon IJ, Kim EY, Kim BY, Cho YS, Chung WH, et al. The auditory and speech performance of children with intellectual disability after cochlear implantation. Acta Otolaryngol. 2013; Jan. 133(1):59–69.

53. Park GY, Moon IJ, Kim EY, Chung EW, Cho YS, Chung WH, et al. Auditory and speech performance in deaf children with deaf parents after cochlear implant. Otol Neurotol. 2013; Feb. 34(2):233–8.

54. Taylor B. Tech savvy, old, contemplative and distorted: four underserved groups who could benefit from OTC products [Internet]. Hearing Health & Technology Matters;2017. [cited 2021 Jul 1]. Available from: https://hearinghealthmatters.org/hearingeconomics/2017/underserved-groups-for-hearing-aids/.
55. Reed NS, Oliver A, Srinivasan NK, Lin FR, Korczak PA. Pilot comparison of adjustment protocols of personal sound amplification products. Semin Hear. 2019; Feb. 40(1):26–36.

56. Holdren JP, Lander E, Press W, Savitz M. Aging America and hearing loss: imperative of improved hearing technologies. Washington (DC): President’s Council of Advisors on Science and Technology;2015.
57. National Academies of Sciences, Engineering, and Medicine. Hearing health care for adults: priorities for improving access and affordability. Washington (DC): National Academies Press;2016.
58. National Institute on Deafness and Other Communication Disorders. Over-the-counter hearing aids [Internet]. Bethesda (MD): National Institute on Deafness and Other Communication Disorders;2020. [cited 2021 Jul 1]. Available from: https://www.nidcd.nih.gov/health/over-counter-hearing-aids.
59. Franck KH, Rathi VK. Regulation of over-the-counter hearing aids: deafening silence from the FDA. N Engl J Med. 2020; Nov. 383(21):1997–2000.

60. The White House. Fact sheet: executive order on promoting competition in the American economy [Internet]. Washington (DC): The White House;2021. [cited 2021 Jul 1]. Available from: https://www.whitehouse.gov/briefing-room/statements-releases/2021/07/09/fact-sheet-executive-order-on-promoting-competition-in-the-american-economy/.
61. Anderson C, Predith A. FDA takes action to deliver lower-cost, innovative hearing aids to millions more Americans [Internet]. The White House President Barack Obama;2016. [cited 2021 Jul 1]. Available from: https://obamawhitehouse.archives.gov/blog/2016/12/07/fda-takes-action-deliver-lower-cost-innovative-hearing-aids-millions-more-americans.
62. Manchaiah V, Taylor B, Dockens AL, Tran NR, Lane K, Castle M, et al. Applications of direct-to-consumer hearing devices for adults with hearing loss: a review. Clin Interv Aging. 2017; May. 12:859–71.

63. Lin FR. Time for a top-down approach to hearing aid affordability and accessibility. Am J Public Health. 2018; Feb. 108(2):166–8.

64. U.S. Food and Drug Administration. Premarket notification 510(k) [Internet]. Silver Spring (ML): U.S. Food and Drug Administration;2020. [cited 2021 Jul 1]. Available from: https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k.
65. American National Standards Institute; Consumer Technology Association. Personal sound amplification performance criteria (ANSI/CTA-2051) [Internet]. Arlington (VA): Consumer Technology Association;2017. [cited 2021 Jul 1]. Available from: https://shop.cta.tech/products/personal-sound-amplification-performance-criteria#:~:text=Qty%3A-,Personal%20Sound%20Amplification%20Performance%20Criteria%20(ANSI%2FCTA%2D2051),amplification%20(OTC%20Hearing%20Aids).
66. Reed NS, Betz J, Kendig N, Korczak M, Lin FR. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA. 2017; Jul. 318(1):89–90.

67. Cho YS, Park SY, Seol HY, Lim JH, Cho YS, Hong SH, et al. Clinical performance evaluation of a personal sound amplification product vs a basic hearing aid and a premium hearing aid. JAMA Otolaryngol Head Neck Surg. 2019; Jun. 145(6):516–22.

68. Seol HY, Kim GY, Kang S, Jo M, Han UG, Cho YS, et al. Clinical comparison of a hearing aid, a personal sound amplification product, and a wearable augmented reality device. Clin Exp Otorhinolaryngol. 2021; Aug. 14(3):359–61.

69. Kim GY, Kim JS, Jo M, Seol HY, Cho YS, Moon IJ. Feasibility of personal sound amplification products in patients with moderate hearing loss: a pilot study. Clin Exp Otorhinolaryngol. 2021; Jan. 29. [Epub]. https://doi.org/10.21053/ceo.2020.02313.

70. Brody L, Wu YH, Stangl E. A comparison of personal sound amplification products and hearing aids in ecologically relevant test environments. Am J Audiol. 2018; Dec. 27(4):581–93.

71. Choi JE, Kim J, Yoon SH, Hong SH, Moon IJ. A personal sound amplification product compared to a basic hearing aid for speech intelligibility in adults with mild-to-moderate sensorineural hearing loss. J Audiol Otol. 2020; Apr. 24(2):91–8.

72. Callaway SL, Punch JL. An electroacoustic analysis of over-the-counter hearing aids. Am J Audiol. 2008; Jun. 17(1):14–24.

73. Mamo SK, Reed NS, Nieman CL, Oh ES, Lin FR. Personal sound amplifiers for adults with hearing loss. Am J Med. 2016; Mar. 129(3):245–50.

74. De Sousa KC, Moore DR, Motlagh-Zadeh L, Myburgh HC, Swanepoel DW. Do smartphone hearing aid apps work. Hear J. 2019; Nov. 72(11):34–7.

75. Amlani AM, Taylor B, Levy C, Robbins R. Utility of smartphone-based hearing aid applications as a substitute to traditional hearing aids. Hear Rev. 2013; Dec. 20(13):16–8.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download