Abstract
Objectives
Owing to the functional and structural complexity of the head and neck area, the reconstruction of defects in these areas is challenging. Free flap surgery has become standard for the reconstruction of the head and neck with improvements in microvascular surgery. The aim of this study was to use the cumulative sum (CUSUM) method to evaluate the learning curve for free-flap head and neck reconstruction performed by a single surgeon.
Methods
We retrospectively reviewed the medical records of 47 patients who underwent free-flap reconstruction from 2017 to 2021. The clinical demographics and surgical outcomes were analyzed. The total operation time was analyzed using the CUSUM method, which is an analytical approach for visualizing patterns in data by converting raw data into an accumulation of deviations from the average value.
Results
CUSUM analysis showed two phases of the learning curve: phase 1 (cases 1–22) and phase 2 (cases 23–47). The operative time in phase 1 (579.9±128.2 minutes) was significantly longer than that in phase 2 (418.6±80.9 minutes) (P<0.001). The re-exploration rate was higher in phase 1 (31.8%) than in phase 1 (4%) (P=0.018). The flap failure rate was higher in phase 1 (9.1%) than in phase 1 (4%), but this difference was not statistically significant (P=0.593).
Due to advances in microvascular surgery, head and neck reconstruction using a free flap has recently become popular. However, when performing head and neck reconstruction after resection of a malignant tumor, the surgeon has to consider both cosmetic aspects and functional aspects such as breathing, swallowing, and vocalization [1,2]. Successful head and neck reconstruction using a free flap requires precise microvascular surgical technique, making it challenging, and the surgeon’s surgical experience plays an important role in the success of the flap. Previous studies have suggested the existence of a learning curve through reports that the success rate of head and neck free-flap reconstruction increases with greater experience [3-5].
The cumulative sum (CUSUM) method was originally developed for monitoring processes in the industrial field [6]. The method involves analyzing continuous data patterns over time, within which changes are detected. The CUSUM technique has also been applied in clinical studies to monitor changes in medical skills and clinical data [7]. To monitor surgical outcomes in pediatric cardiac surgery, de Leval et al. [8] and Steiner et al. [9] used the CUSUM curve for the first time. However, medical professionals have not used the CUSUM method to analyze the learning curve of flap surgery for head and neck reconstruction. Evaluating and reporting surgical outcomes over time as a surgeon gains experience will help alleviate the burden for many surgeons willing to conduct free flap reconstruction. Therefore, we aimed to evaluate the learning curve for free-flap head and neck reconstruction surgery using the CUSUM method.
We retrospectively reviewed the medical records of 47 patients who underwent free-flap reconstruction of the head and neck between August 2017 and January 2021. All procedures including wide mass excision with or without neck dissection followed by free-flap reconstruction were performed by a single surgeon (DYL). Clinical features, operation time, hospital stay, flap failure rate, re-exploration rate, and complications were analyzed according to the time period.
This study was approved by the Institutional Review Board of Seoul National University Boramae Medical Center (IRB No. 30-2021-64). Informed consent from patients was waived as this was a retrospective study. All procedures were performed under general anesthesia in the following order: resection of the tumor, recipient vessel preparation, flap harvest and insetting, and microvascular anastomosis. The flap was selected based on the surgeon’s expertise and the features of the defect. All microvascular procedures were performed using a binocular operating microscope (Carl Zeiss Meditec, Jena, Germany). All patients underwent intensive care unit care for the first 24 hours after surgery. The flap was monitored using an infrared thermometer, the color of the flap, and pinprick.
The CUSUM method was used to analyze the learning curves; it utilizes a quantitative approach that measures the differences between the individual data value and the average value of all data [10]. First, 47 cases of free-flap reconstruction were chronologically ordered according to the date of surgery. When we define the operation time of each case as “Xi” and the average operation time as “μ,” and let the CUSUM of the “n” cases sequentially be CUSUMoptime_n, the CUSUMoptime_n of each case can be defined as follows:
For example, if the CUSUM of the first free-flap reconstruction is CUSUMoptime1, the total operation time of the first patient was 615 minutes, and the average total operation time was 494.11 minutes. Therefore, CUSUMoptime1 is 120.89. The second procedure time was 532 minutes, so CUSUMoptime2 became 158.78, which is the value of CUSUMoptime1, which is 120.89, plus 37.89 (532–494.11 minutes). Thus, each CUSUMoptime_n can be calculated using the formula for Microsoft Excel 2010 (Microsoft, Redmond, WA, USA).
The slope of the CUSUM curve represents the trend of learning outcomes, and the area where the slope is stabilized is regarded as the breakthrough of the learning curve [11]. The moving average curve is the moving average of the operation time converted into the graph. The X axis displays the case consequently, and the Y axis represents the mean operating time which was a group of five cases.
Differences in categorical variables were compared using the Pearson chi-square test or Fisher’s exact test, and the means of continuous variables were compared using Student t-test. All data were analyzed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). Continuous data are presented as mean±standard deviation. In all cases, a P<0.05, was considered statistically significant.
The average age of all patients was 58.4±12.6 years. As shown in Fig. 1, the curve in the CUSUM analysis showed a steeply rising slope from case 1 to 22, and then rapidly declined after case 23. Therefore, the sample was divided into two phases (Fig. 1). The interval from case 1 to case 22 was defined as phase 1, and the interval from case 23 to case 47 (the last case) was defined as phase 2.
The differences in the clinical features between phases 1 and 2 are presented in Table 1. There were no statistically significant differences in the age group, primary site, stage of cancer, or previous treatment between phase 1 and phase 2 (Table 1). The operation time was significantly longer in phase 1 than in phase 2 (P<0.001). The re-exploration rate was also significantly higher in phase 1 than in phase 2 (P=0.018). The length of hospital stay after surgery, estimated blood loss, flap failure rate, period until decannulation, and period until return to oral diet were lower in phase 2 than in phase 1, but without statistical significance (Table 2). The five-data-point moving average curve showed that the operation time stabilized as the number of cases increased (Fig. 2).
When comparing the operation time between phases according to flap type and reconstructed site, the operation time of phase 1 for both radial forearm free flaps and anterolateral thigh free flaps was significantly longer than that of phase 2 (P=0.009 and P<0.001, respectively) (Table 3). In addition, the operation time of phase 1 was significantly longer than that of phase 2 for oral cavity or oropharyngeal reconstruction and larynx or hypopharyngeal reconstruction (P=0.002 and P=0.019, respectively).
The learning curve is a result of the surgeon gradually becoming comfortable with the procedure and success in more difficult cases, and it also reflects the surgeon’s increased knowledge of the new technique, technological changes to the technique, and the improvements in support staff and perioperative treatment [12,13]. Many factors affect the success of free flap surgery, including surgical procedures at the recipient and donor sites, meticulous microvascular anastomosis, the location of the vascular pedicle, and post-surgical monitoring [14]. The surgeon’s surgical experience is the most significant factor in preventing free flap failure [15]. As a result, for new microvascular surgeons, the learning curve is critical.
This study demonstrated that operation time, used as a marker for operative competency, was correlated to components of the surgeon’s learning process using CUSUM analysis. The learning curve could be divided into two phases: before and after case 22. Similarly, in a previous study using the CUSUM method for colorectal surgery, the learning curve phase was relatively clearly divided into three phases [10]. However, studies on the learning curve of free-flap head and neck reconstruction surgical procedures are not common. Previous studies have used the flap failure rate, salvage rate, and complication rate to evaluate the learning curve. However, none have used CUSUM analysis to determine the learning curve in head and neck free-flap reconstruction surgery [16-19]. In addition to operation time, we tried to define the phases by risk-adjusted CUSUM analysis for flap failure. However, the number of total cases was small and the failure rate was low; thus, phases could not be defined through risk-adjusted CUSUM.
The CUSUM technique was developed in the industrial sector to track output and identify areas for improvement. The medical profession began using this approach to analyze the learning curve for surgical procedures in the 1970s [20,21]. Unlike other analytical methods, the CUSUM method quantifies fluctuations based on a predetermined threshold value, such as the average. The CUSUM method accounts for even small changes between a sequential event value and a predetermined threshold value, better reflecting the degree of change.
Due to their functional and structural complexity, reconstruction of head and neck defects is always challenging. Since the late 1970s, the pectoralis major myocutaneous flap has become the most important flap due to its ease of operation [22]. It is simple to use because it does not require vascular surgery, but the flap is extremely thick, and there are multiple problems in the functional reconstruction of the oral cavity or the esophagus. The free flap technique has been actively adapted to the head and neck area since the radial forearm free flap was reported in 1981 [23]. The radial forearm free flap is based on the radial artery. A large-diameter artery (≥2.5 mm) can be used, with two accompanying veins and cephalic veins. Additionally, the thickness of the flap is 2–3 mm. Due to its slenderness, it has the advantage of making it possible to construct the desired shape easily, for which reason it has become the most commonly used flap. In 1984, Song et al. [24] reported that the advantages of the anterolateral thigh flap include the possibility of obtaining a large flap that is easy to raise, with an inconspicuous donor site. These free flaps led to significant improvements in functional recovery after surgery in patients with head and neck malignancies. In addition, reconstructive surgery using these flaps is expected to become increasingly important due to their benefits for advanced oropharyngeal and laryngeal cancers [25].
Previous studies have shown that microvascular surgeons reported better outcomes with more experience [3,26]. Urken et al. [3] showed an 89% success rate with 75 free flaps and a 96% success rate after 125 additional cases. After conducting 64 microvascular free flaps, Watkinson and Breach [4] reported a 79% success rate, and after 77 other cases, the success rate increased to 95%. Godina [5] reported a 74% success rate after 100 free flaps and a 96% success rate after 100 other free flaps. Harashina [27] also showed a 75% success rate in the first 3 years after performing free flaps, which increased to 97% in the next 5 years. These findings are comparable to our results, which showed that as experience increased, outcomes improved.
In this study, the re-exploration rate was analyzed as a factor that may have a difference between phases, and the re-exploration rate was indeed found to be significantly higher in phase 1 than in phase 2. Phase 1, corresponding to the first 22 cases, required seven re-explorations. Salvage of the flap was possible in five cases by hematoma evacuation and pharyngostoma construction, but rescue failed in two cases with arterial insufficiency. In contrast, in the second stage, only one of the 21 patients underwent re-exploration due to arterial insufficiency. A re-exploration was conducted if it was determined that the flap condition could lead to flap failure, such as in cases with findings suggestive of vascular compromise. Therefore, re-exploration was performed promptly after flap surgery, which may have been why the hospital stay was not substantially longer in the first phase, even though re-exploration was performed more often.
In this study, although the clinical features of each phase did not differ significantly from each other, it was confirmed that the total operation time significantly decreased with experience. This suggests that the time required for reconstruction was shortened, as there was no difference in the time of tumor excision and neck dissection between the two phases. Compared to phase 1, the standard deviation of operation time was lower in phase 2, and the average curve showed that the operation time stabilized with increasing experience. We believe that the learning curve reflects important factors in the surgeon’s mastery of free-flap head and neck reconstruction, such as skilled surgical technique of microvascular anastomosis, appropriate recipient vessel selection, conceptualization of the spatial relationships of the flap for manipulation and insetting, and planning of pedicle alignment to prevent vessel kinking or extrinsic compression. The reason that the success rate was 97% despite little experience may have been that the surgeon already had more than 12 years of experience with other head and neck surgical procedures and had participated in various head and neck reconstructions as an assistant.
Our study has several limitations. First, it is difficult to generalize the results, since this was a retrospective study based on data from a single surgeon. Free-flap head and neck reconstruction surgery consists of several steps: flap harvest, insetting, and microvascular anastomosis. In this study, the analysis of each step was limited. However, using the CUSUM method, two different phases within the learning curve could be relatively well distinguished. Prospective studies that include several head and neck surgeons should be conducted in the future.
Using the CUSUM method, it was found a surgeon who had experience with head and neck surgery required approximately 20 cases to show stable performance in head and neck free-flap reconstruction. However, for a surgeon who does not have sufficient experience in head and neck surgery and is conducting head and neck free-flap reconstruction for the first time, the learning curve may be longer than 20 cases. During the learning period of novice surgeons, efforts to increase skills under the supervision of a skilled instructor are essential.
REFERENCES
1. Kim H, Jeong WJ, Ahn SH. Results of free flap reconstruction after ablative surgery in the head and neck. Clin Exp Otorhinolaryngol. 2015; Jun. 8(2):167–73.

2. Hurvitz KA, Kobayashi M, Evans G. Current options in head and neck reconstruction. Plast Reconstr Surg. 2006; Oct. 118(2):122e–133e.

3. Urken ML, Weinberg H, Buchbinder D, Moscoso JF, Lawson W, Catalano PJ, et al. Microvascular free flaps in head and neck reconstruction: report of 200 cases and review of complications. Arch Otolaryngol Head Neck Surg. 1994; Jun. 120(6):633–40.

4. Watkinson JC, Breach NM. Free flaps in head and neck reconstructive surgery: a review of 77 cases. Clin Otolaryngol Allied Sci. 1991; Aug. 16(4):350–3.

5. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986; Sep. 78(3):285–92.

6. Barnard GA. Control charts and stochastic processes. J R Stat Soc Series B Stat Methodol. 1959; 21(2):239–71.

7. Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics. 2000; Dec. 1(4):441–52.

8. de Leval MR, Francois K, Bull C, Brawn W, Spiegelhalter D. Analysis of a cluster of surgical failures. Application to a series of neonatal arterial switch operations. J Thorac Cardiovasc Surg. 1994; Mar. 107(3):914–23.

9. Steiner SH, Cook RJ, Farewell VT. Monitoring paired binary surgical outcomes using cumulative sum charts. Stat Med. 1999; Jan. 18(1):69–86.

10. Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011; Mar. 25(3):855–60.

11. Park EJ, Kim MS, Kim G, Kim CH, Hur H, Min BS, et al. Long-term oncologic outcomes of laparoscopic right hemicolectomy during the learning curve period: comparative study with cases after the learning curve period. Surg Laparosc Endosc Percutan Tech. 2015; Feb. 25(1):52–8.
12. Tekkis PP, Fazio VW, Lavery IC, Remzi FH, Senagore AJ, Wu JS, et al. Evaluation of the learning curve in ileal pouch-anal anastomosis surgery. Ann Surg. 2005; Feb. 241(2):262–8.

13. Heo SJ, Park CM, Kim JS. Learning curve of septoplasty with radiofrequency volume reduction of the inferior turbinate. Clin Exp Otorhinolaryngol. 2013; Dec. 6(4):231–6.

14. Ban MJ, Na G, Ko S, Kim J, Heo NH, Choi EC, et al. Externally monitored versus conventional buried flaps in laryngopharyngeal reconstruction. Clin Exp Otorhinolaryngol. 2021; Nov. 14(4):407–13.

16. Blackwell KE, Brown MT, Gonzalez D. Overcoming the learning curve in microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1997; Dec. 123(12):1332–5.

17. Demir A, Kucuker I, Keles MK, Demirtas Y. The effect of learning curve on flap selection, re-exploration, and salvage rates in free flaps: a retrospective analysis of 155 cases. Microsurgery. 2013; Oct. 33(7):519–26.

18. Klosterman T, Siu E, Tatum S. Free flap reconstruction experience and outcomes at a low-volume institution over 20 years. Otolaryngol Head Neck Surg. 2015; May. 152(5):832–7.

19. Ratnagiri R, Jena S, Parvathi P, Srikanth R, Raju G. Reconstruction in head-and-neck cancers: analysis of the learning curve. Natl J Maxillofac Surg. 2018; Jul-Dec. 9(2):191–5.

20. Chaput de Saintonge DM, Vere DW. Why don’t doctors use cusums. Lancet. 1974; Jan. 1(7848):120–1.
21. Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977; May. 296(18):1044–5.

22. Ariyan S, Cuono CB. Myocutaneous flaps for head and neck reconstruction. Head Neck Surg. 1980; Mar-Apr. 2(4):321–45.

23. Yang GF, Chen PJ, Gao YZ, Liu XY, Li J, Jiang SX, et al. Forearm free skin flap transplantation: a report of 56 cases. 1981. Br J Plast Surg. 1997; Apr. 50(3):162–5.
24. Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984; Apr. 37(2):149–59.

25. Park JO, Park YM, Jeong WJ, Shin YS, Hong YT, Choi IJ, et al. Survival benefits from surgery for stage IVa head and neck squamous cell carcinoma: a multi-institutional analysis of 1,033 cases. Clin Exp Otorhinolaryngol. 2021; May. 14(2):225–34.

Fig. 1.
Total operation (OP) time and cumulative sum (CUSUM) curves of free-flap reconstruction surgery. The light line is a plot of a second-order polynomial with the equation CUSUM=–3.0551×(case number)2+139.64×(case number)+40.482; R2=0.9325. At case 22, the gray line represents the breakthrough point.
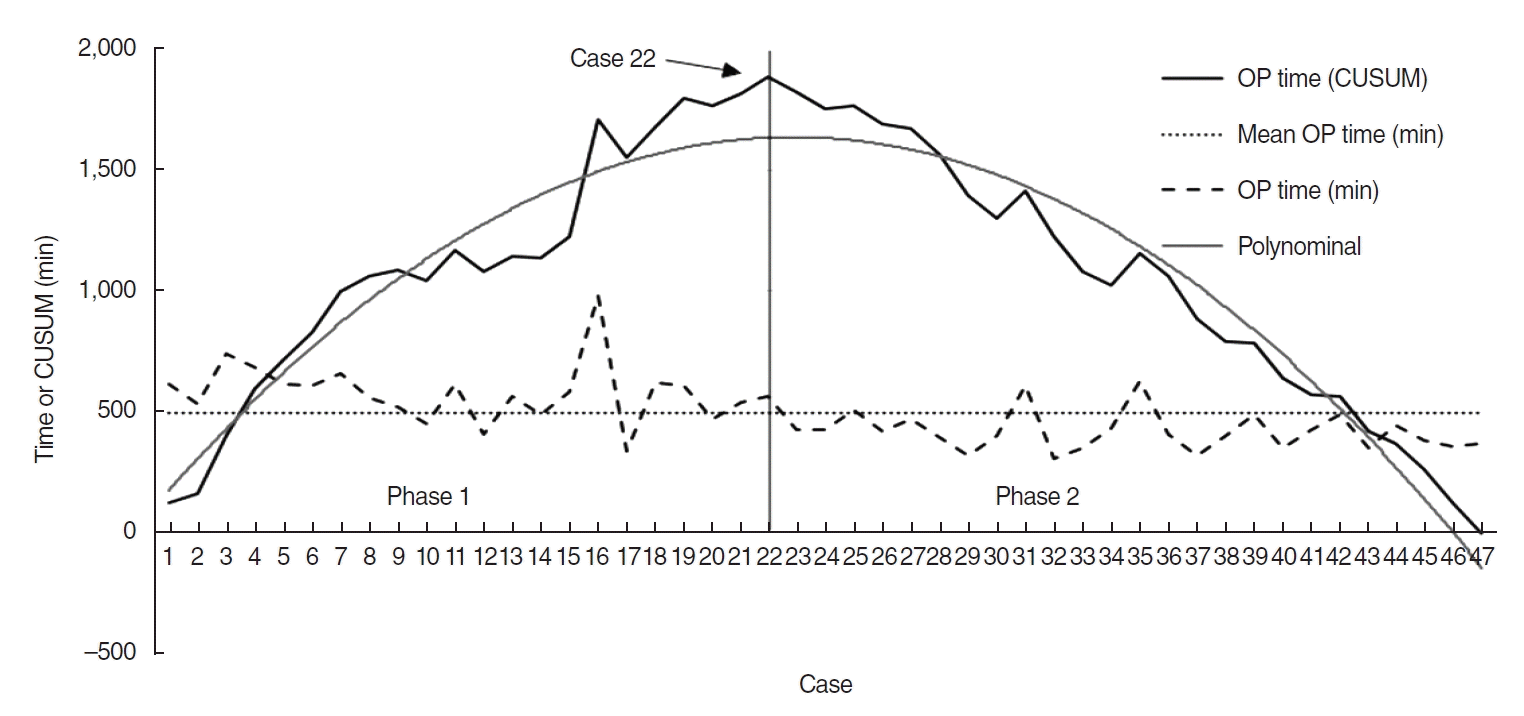
Fig. 2.
Five-data moving average curve (MAC) according to total operation time of free-flap reconstruction surgery. 5D MAC, five-datapoint MAC.
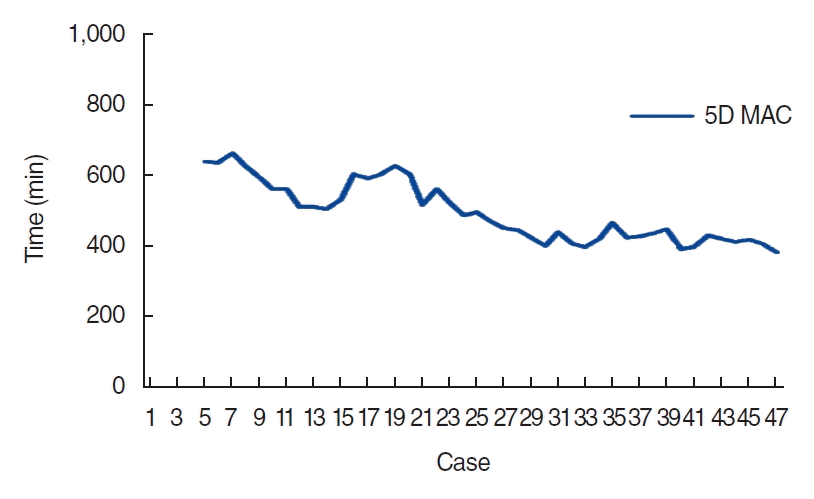
Table 1.
Interphase comparisons of patients’ clinical features
Table 2.
Interphase comparison of intraoperative parameters and surgical outcomes
| Variable |
CUSUM phase |
P-value | |
|---|---|---|---|
| Phase 1 (case 1–22) | Phase 2 (case 23–47) | ||
| Operation time (min) | 579.9±128.2 | 418.6±80.9 | <0.001 |
| Postoperative hospital stay (day) | 25.3±18.4 | 19.4±14.5 | 0.255 |
| Estimated blood loss (mL) | 720.9±652.9 | 564±424.2 | 0.328 |
| Flap failure | 2 (9.1) | 1 (4) | 0.593 |
| Re-exploration | 7 (31.8) | 1 (4) | 0.018 |
| Decannulation (day)a) | 10.9±9.5 | 7.4±4 | 0.246 |
| Return to oral diet (day)b) | 18.0±19.1 | 13.5±9.1 | 0.405 |
Table 3.
Interphase comparison of operation time according to flap type and reconstruction site
| Variable |
CUSUM phase |
P-value | |
|---|---|---|---|
| Phase 1 (case 1–22) | Phase 2 (case 23–47) | ||
| Flap type | |||
| RFFFa) | 615.3±76.6 | 438±130.1 | 0.009 |
| ALTFFb) | 518.8±87.9 | 412.8±56.4 | <0.001 |
| Reconstruction site | |||
| Oral cavity, oropharynxc) | 587.5±156.8 | 427.6±90.2 | 0.002 |
| Larynx, hypopharynxd) | 566.6±131.1 | 403.5±66.6 | 0.019 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download