INTRODUCTION
Turbinate hypertrophy is one of the most common causes of chronic nasal obstruction. A recent study showed that 10%–20% of the global population suffers from nasal obstruction due to allergic rhinitis, which causes a significant deterioration in the quality of life [
1]. The common causes of turbinate hypertrophy include allergic rhinitis, vasomotor rhinitis, and chronic hypertrophic rhinitis. Although transient symptom relief can be achieved through medical treatment with agents such as antihistamines, oral vasoconstrictors, or intranasal corticosteroids, a significant proportion of patients require surgical volume reduction [
2].
Techniques for reducing turbinate hypertrophy include microdebrider-assisted turbinoplasty, radiofrequency turbinoplasty, and turbinate resection. Most of these techniques are effective; however, the outcomes differ depending on the degree of technical invasiveness [
3]. Turbinoplasty is usually performed as an adjunctive procedure during septoplasty, which is generally performed inside an operating theater. Under those circumstances, techniques providing better outcomes may be preferred, as bleeding can be easily controlled. However, office-based turbinoplasty is becoming more common, leading to an unmet need for minimally invasive turbinoplasty.
In recent years, high-intensity focused ultrasound (HIFU) has been used for the treatment of benign lesions in obstetrics, gynecology, and urology. HIFU is a noninvasive treatment that effectively coagulates and necrotizes targets tissues (mainly solid tumors) that are located deep within the body without damaging the surrounding tissue [
4]. It is applied at a frequency of 0.8–3.0 MHz depending on the type of instrument used [
5]. Ultrasonic waves within this range can reach the tissue and rapidly increase the target focal temperature to 80°C or higher, leading to coagulation and necrosis. Furthermore, the ultrasonic waves also cause vibration in the target tissue, resulting in compression and thinning of molecular structures [
6]. High-energy HIFU can be amplified and targeted to a very small focal area without damaging the surrounding tissue. Unlike traditional surgery for tissue removal, HIFU does not physically penetrate the surface tissue. In light of the low likelihood of epithelial injury, minimal bleeding and rapid wound healing are expected. As the factors influencing hypertrophy include thickened mucosa with enlarged venous sinusoids [
7], we hypothesized that tissue reduction or ablation with HIFU, which involves a minimal injury, may be a good treatment option for refractory chronic rhinitis. Therefore, the goal of this study was to design and develop an optimal HIFU device for the treatment of turbinate hypertrophy.
This study aimed to confirm that HIFU leads to an appropriate response when used for the treatment of turbinate hypertrophy causing nasal obstruction in patients with chronic rhinitis. Thus, the present study describes the development of a HIFU device for turbinoplasty. The diameter and focal depth were determined, and an efficacy study was then conducted in an animal model.
DISCUSSION
Hypertrophy of the IT is an important anatomical factor leading to chronic nasal obstruction in patients with chronic rhinitis. The factors underlying hypertrophy include mucosal and bone hypertrophy with the enlargement of venous sinusoids [
7]. These pathophysiological findings can be corrected by total/partial turbinate resection or turbinoplasty, for which various techniques have been developed. The classical technique involves raising a submucosal flap and partially removing the submucosal tissues. Recently, remarkably simple radiofrequency ablation equipment and microdebriders have been developed for turbinoplasty. A recent meta-analysis showed that both radiofrequency and microdebrider-assisted turbinoplasty were equally effective and achieved significant improvements in cases of nasal obstruction [
10].
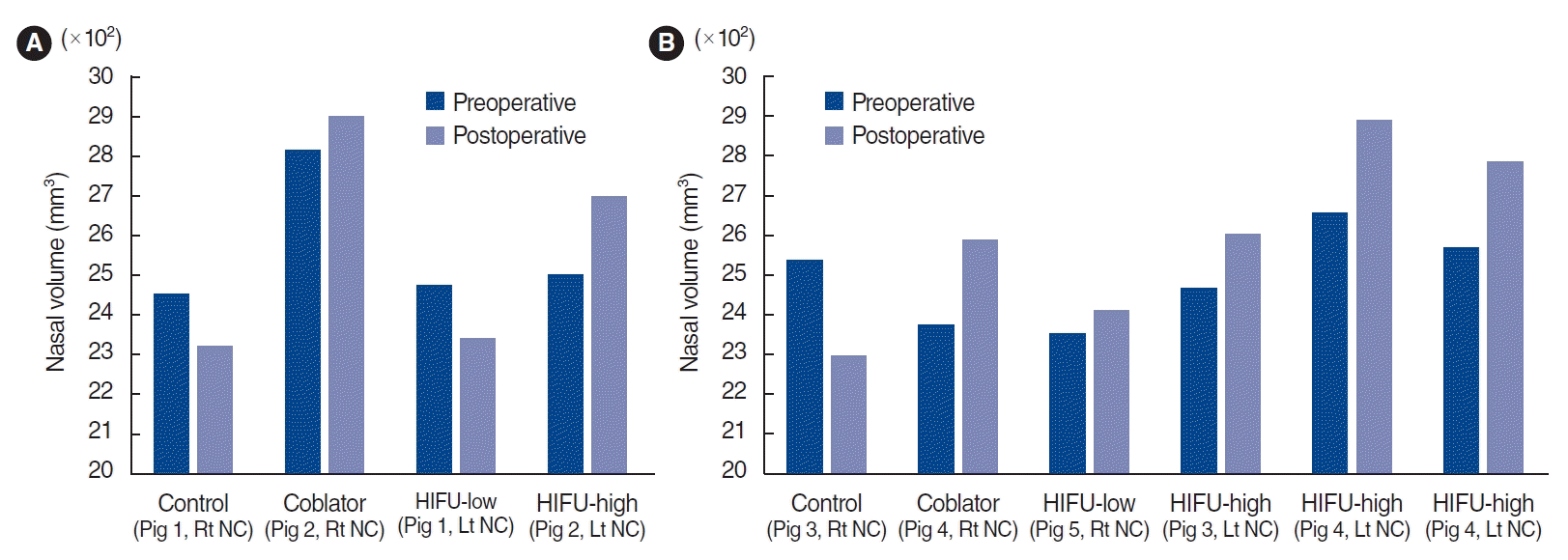
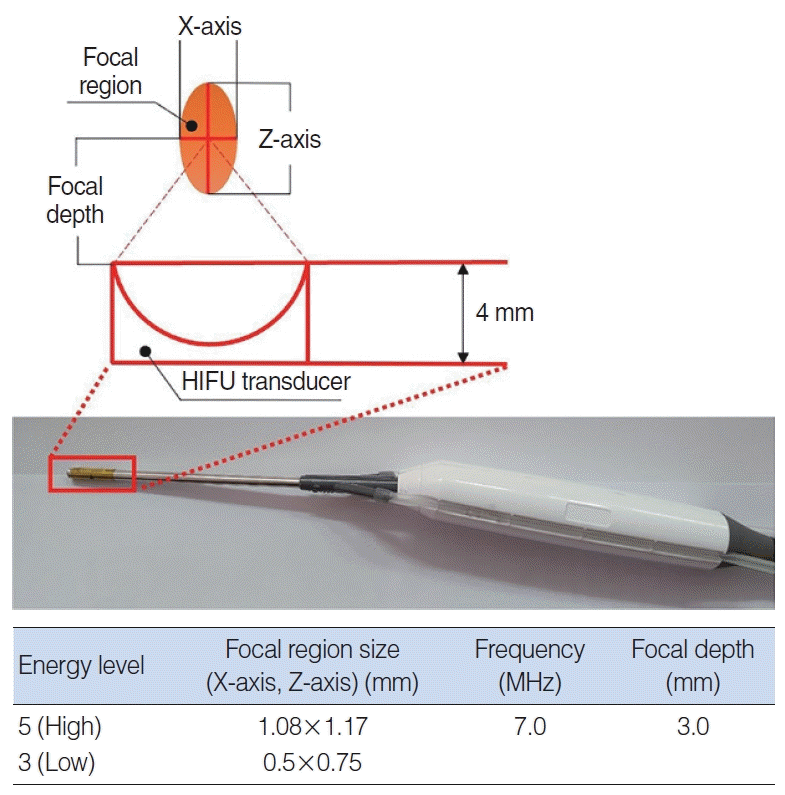
The current study described the development of a HIFU prototype for turbinoplasty, which was based on evaluations of the diameter and focal depth of the nasal cavity. HIFU turbinoplasty showed no complications such as bleeding or scar formation and efficiently reduced the turbinate size in a porcine model. Unlike a radiofrequency device, which requires saline injection, pre-treatment manipulation of the turbinate is not needed with our HIFU-assisted technique. Therefore, the device should fit the existing space in the nasal cavity for prompt device manipulation and visualization of the nasal cavity. The HIFU prototype device was designed considering the available range of the nasal cavity airway space. The mucosal depth was also considered to be effective for the HIFU treatment, as a HIFU wave beyond this depth would be transmitted through the bone, potentially reducing the treatment efficacy, whereas a shallower depth could lead to epithelial damage. In this study, the space in the nasal cavity was approximately 2.65 mm. For technical reasons, a diameter of at least 4 mm was needed to deliver sufficient energy, which would lead to compression of the turbinate mucosa with a difference of approximately 1.35 mm from the thickness (4.23–6.17 mm). Therefore, the projection depth was adjusted to 3 mm.
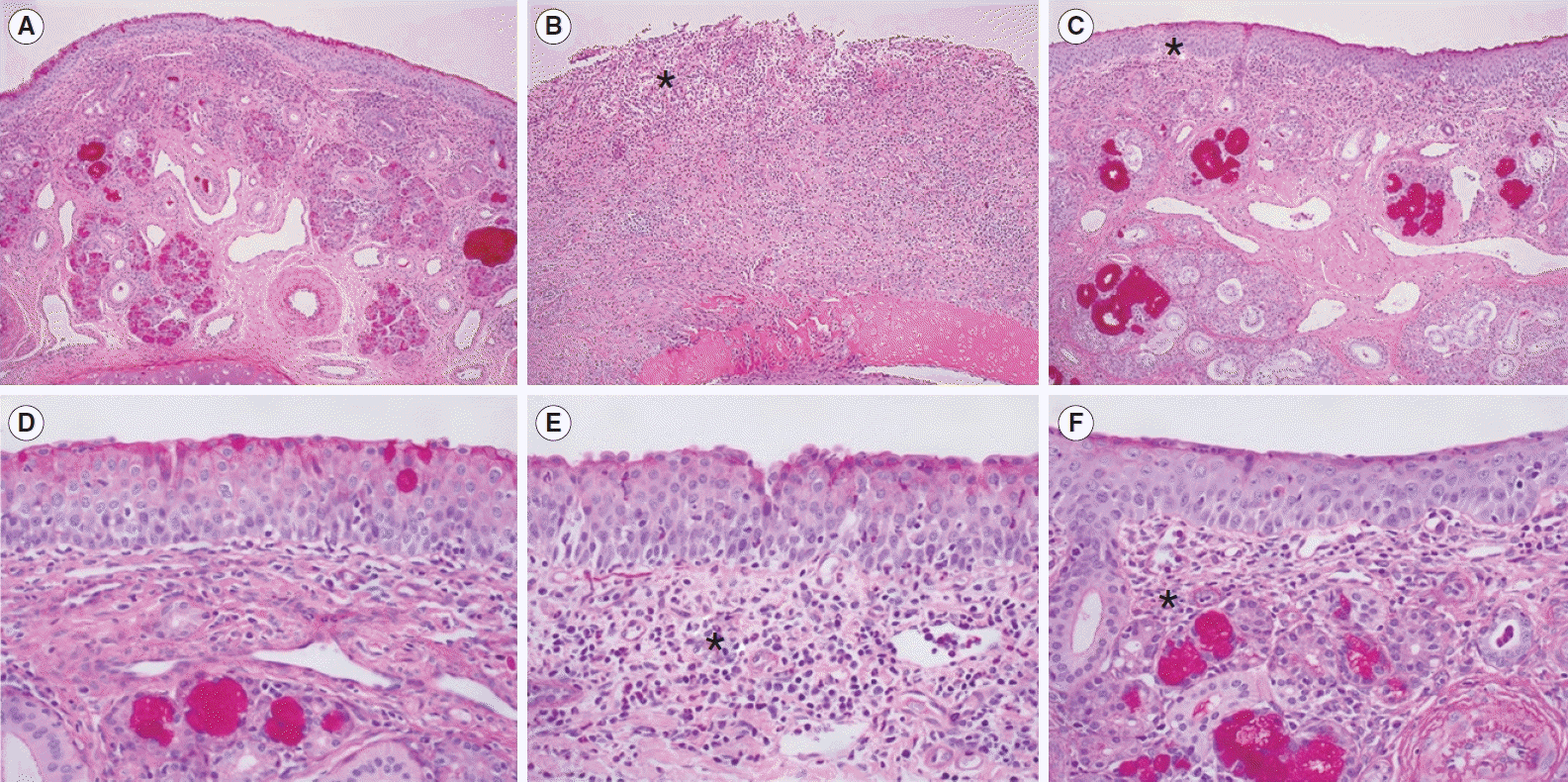
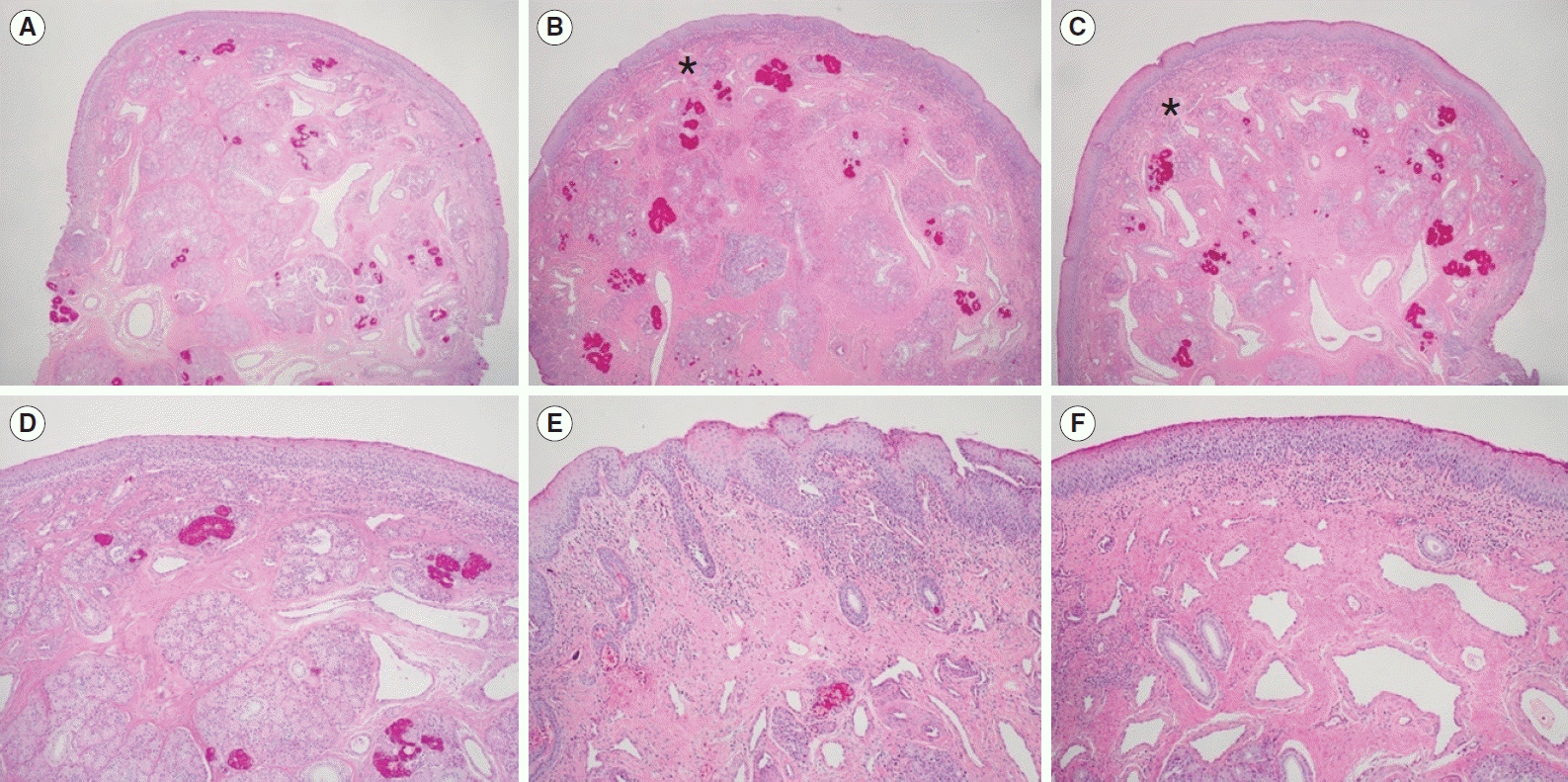
The histological examinations showed an increased submucosal polymorphonuclear cell count, decreased glandular structure, and increased fibrosis in the HIFU-treated group. Increased fibrosis and the replacement of large venous sinusoids by veins are well-known histological changes observed in radiofrequency turbinoplasty [
11]. We also found that histological changes in the IT mucosa were affected by the HIFU intensity, as demonstrated by the higher degree of polymorphonuclear cell infiltration after 1 week of treatment in the HIFU-high group than in the HIFU-low group. Polymorphonuclear cell infiltration with maintenance of the tissue architecture is the hallmark of coagulation necrosis, which may be further replaced by tissue fibrosis [
12]. The higher degree of submucosal polymorphonuclear cell infiltration appeared to increase the nasal airway space after 1 week in the HIFU-high group to a greater extent than in the HIFU-low group. However, the difference between the HIFU-high and HIFU-low groups was not significant after 4 weeks. Our results indicate that both levels of HIFU treatment were effective for IT hypertrophy after 4 weeks of treatment.
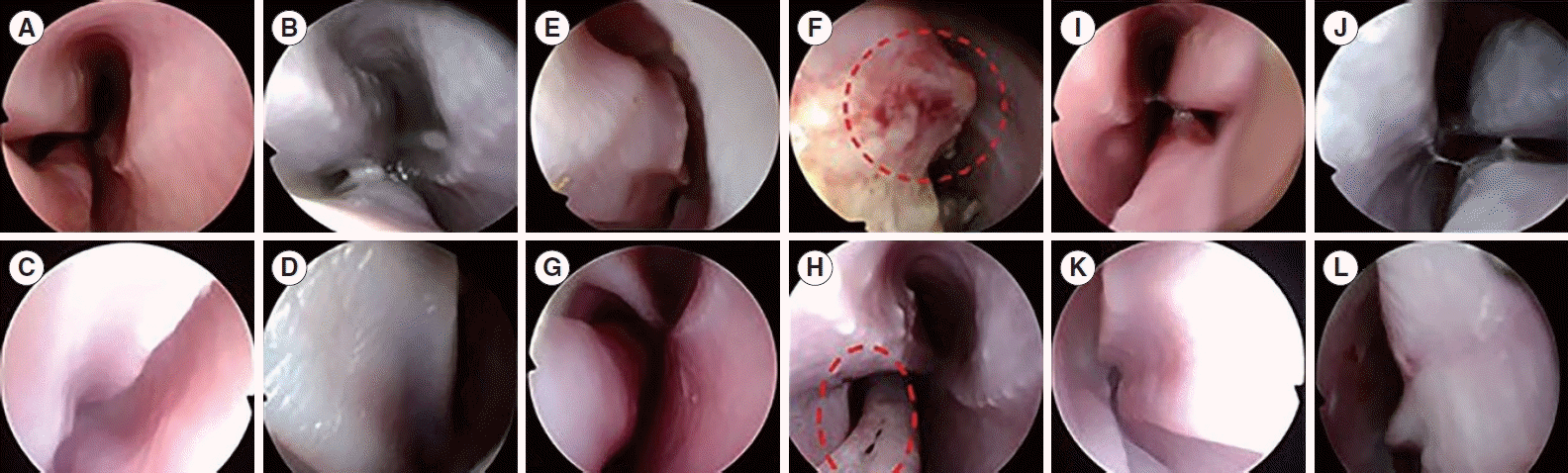
In our study, the efficacy of HIFU turbinoplasty was similar to that of radiofrequency turbinoplasty. However, HIFU turbinoplasty was more beneficial for treatment of the IT than radiofrequency turbinoplasty. First, on endoscopic and histological examinations, the HIFU group showed minimal epithelial layer damage and no bleeding, while the radiofrequency group showed gross and microscopic tissue surface damage. Second, HIFU was performed in a local area without additional preparation such as saline injection. Therefore, the convenience and utility of the HIFU device indicate its potential for use in the outpatient setting.
The depth of our prototype was 3 mm, which was larger than the medial MT in our animal models. However, volume reduction was achieved in all the animal models 4 weeks after HIFU turbinoplasty. Multiple explanations can be proposed for this finding. First, since the energy transmission seemed to be focused on the turbinate bone, which has a high absorption rate, only a small portion of the ultrasound energy may have been transmitted to the soft tissue. However, thermal energy can be secondarily transmitted to the adjacent periosteum from the bone, which may have affected the turbinate mucosa [
13]. Additionally, the presence of an oval-shaped healing region (1.08×1.17 mm) from the center indicates partial energy transmission to the turbinate mucosa. The effect of the focal depth on the efficacy of HIFU turbinoplasty needs to be further evaluated.
Our study has several limitations. First, a quantitative analysis was not performed to compare the histological changes. Second, while the nasal cycle is present in swine [
14,
15], we did not consider it when measuring the airway space by CT scans, and changes in the nasal airway space may have been affected by the nasal cycle [
16]. Third, the HIFU turbinoplasty demonstrated only short-term efficacy. Therefore, additional studies using swine models may be warranted. However, this is the first study to demonstrate the efficacy of HIFU turbinoplasty in animal models by showing both radiological and histological changes after HIFU treatment. Considering the safety of HIFU turbinoplasty, human clinical studies may be initiated with our device.
In conclusion, in our study, nasal endoscopic findings showed better mucosal preservation in HIFU-treated turbinates than in radiofrequency-treated turbinates. The volume of the nasal cavity calculated by CT scans was higher in the HIFU-treated turbinates than in the non-treated turbinates, and this effect was similar to the changes observed in radiofrequency-treated turbinates. The histopathological findings of HIFU-treated turbinates revealed the induction of focal tissue coagulation of the submucosal layer, while preserving the mucosal layer. Therefore, HIFU turbinoplasty may be a useful technique to reduce IT hypertrophy without complications.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download