서론
Table 1.
| Study | Study design | Number of subjects/age | Audiologic assessment | Cognitive function assessed | Results |
|---|---|---|---|---|---|
| Lin, et al. [3] | Prospective, longitudinal (median follow-up of 11.9 years) | 639 / 36-90 years | Pure-tone average of hearing thresholds at 0.5, 1,2, and 4 kHz | incidence of all-cause dementia and Alzheimer disease | The risk of incident all-cause dementia increased loglinearly with the severity of baseline hearing loss (1.27 per 10 db loss; 95% CI, 1.06-1.50) |
| Lin, et al. [4] | Prospective, longitudinal (followed up for 6 years) | 1984 / 77.4 years (mean) | Pure-tone average of hearing thresholds at 0.5, 1,2, and 4 kHz | 3MS (measuring global function) | Compared to those with normal hearing, individuals with hearing loss (pure-tone average >25 dB) had a 24% (HR, 1.24; 95% CI, 1.05-1.48) increased risk for incident cognitive impairment |
| Digit Symbol Substitution test (measuring executive function) | |||||
| # Incident cognitive impairment: defined as 3MS score of less than 80 or a decline in 3MS score of more than 5 points from baseline | |||||
| Gallacher, et al. [5] | Prospective, longitudinal (followed for 1 7 years) | 1057 / 56.1 years (mean) | Pure-tone average of hearing thresholds at 0.5, 1,2, and 4 kHz | Incident dementia: DSM-IV criteria, including standard criteria for vascular dementia and for Alzheimer disease | Increased risk of dementia (OR, 4.07; 95% CI, 2.21-7.50 per 10-dBA rise in usual PTA) |
| Cognitive decline: cognitive test battery | Further adjustment for social class, anxiety, and premorbid cognitive ability attenuated the association (OR, 2.67; 95% CI, 1.38-5.18) | ||||
| Amieva, et al. [6] | Prospective, longitudinal (25-year follow-up) | 3670 / 73.8 years (mean) at no hearing loss group (n=2394), 76.7 years (mean) at self-reported moderate hearing loss group (n=1139), 81.7 years (mean) at Self-reported major hearing loss group (n=137) | Questionnaire assessing self-perceived hearing loss | MMSE | Self-reported hearing loss was significantly associated with lower baseline MMSE score (β=-0.69, p0.001) and greater decline during the 25-year follow-up period (β=-0.04, p=0.01) independent of age, sex, and education. A difference in the rate of change in MMSE score over the 25-year follow-up was observed between participants with hearing loss not using hearing aids and controls (β=-0.06, p0.001). In contrast, subjects with hearing loss using a hearing aid had no difference in cognitive decline (β=0.07, p=0.08) from controls |
| Deal, et al. [7] | Prospective, longitudinal (over 9 years) | 1889 in Health ABC study, 721 in CVS / 70-79 years in Health ABC study, 72-81 years in CVS | Pure-tone average of hearing thresholds at 0.5, 1,2, and 4 kHz | Dementia: defined using a prespecified algorithm incorporating medication use, hospital records, and neurocognitive test scores | Three-hundred eighty seven (20%) participants had moderate/severe HI, and 716 (38%) had mild HI. After adjustment for demographic and cardiovascular factors, moderate/severe audiometric HI (vs. normal hearing) was associated with increased risk of incident dementia over 9 years (HR, 1.55; 95% CI, 1.10-2.19). |
CI, confidence interval; HR, hazard ratio; 3MS, Modified Mini-Mental State Examination; OR, odds ratio; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; PTA, puretone average threshold; MMSE, Mini-Mental State Examination; ABC, Aging, and Body Composition; CVS, Health ABC Cognitive Vitality Substudy; HI, heairng impiarment
본론
노인에게 난청과 인지기능 저하의 연관성
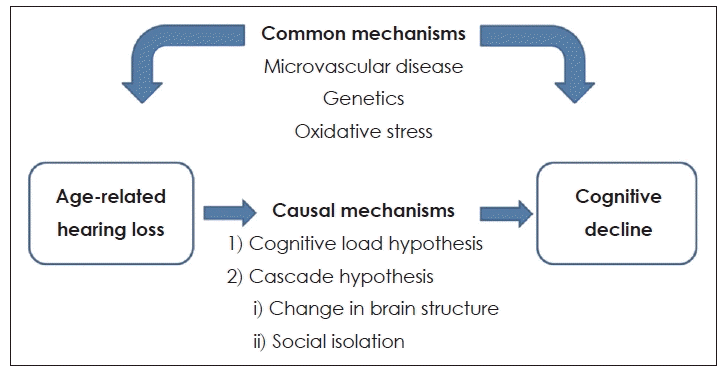 | Fig. 1.Conceptual model of the association of age-related hearing loss with cognitive decline. Adapted from Chung. Korean J Otorhinolaryngol-Head Neck Surg 2020;63(4):145-53[9]. |
노인 환자에서 인공와우 수술 후 결과
노인 환자에서 인공와우 수술이 인지기능에 미치는 영향
Table 2.
| Study | Number of subjects | Age | Duration of deafness | Cognitive outcome measure | Outcome |
|---|---|---|---|---|---|
| Ambert-Dahan, et al. [48] | 18 (postlingual deafness) | 64 (±3.5) (range: 23-83) | 6.5 (±2.1) years (range: 0.3-35 years) | Assessed prior to and 12 months after implantation by means of 2 cognitive screening tests, the CODEX and the MoCA | 4 of the 8 participants with abnormal scores before implantation, improved their cognitive performance into the normal range 12 months after implantation. On the other hand, 3 of the 10 participants with normal preoperative cognitive scores demonstrated a decrease in performance, but remained in the normal range. |
| Castiglione, et al. [49] | CI recipient (n=15) vs. normal hearing control (n=20) | Median: 71 (range: 67-75) vs. median: 70 (range: 65-80) | Not reported | Assessed before and 1 year after implantation using MoCA, GDS | Prior to implantation, the mean MoCA score was 25.7 (±3.6) and after implantation 27.2 (±3.7), which was a significant improvement (p0.01). The postimplantation MoCA scores of the CI recipients did not significantly differ from the MoCA score of the NH listeners. |
| Cosetti, et al. [50] | 7 (postlingual deafness) | 73.6 (±5.82) (range: 67-81) | 29 years (range: 8-53 years) | Assessed prior to and average 3.7 years (range: 2-4.1 years) after implantation using an extensive cognitive test battery comprising the WASI, TMT parts A and B, controlled oral word association test, the Boston naming test and the RBANS | Improvements after CI were observed in 14 (70%) of all subtests administered. Declines occurred in 5 (25%) subtests. In 55 individual tests (43%), post-CI performance improved compared to a patient’s own performance before implantation. Of these, 9 (45%) showed moderate or pronounced improvement. Overall, improvements were largest in the verbal and memory domains. Logistic regression demonstrated a significant relationship between speech perception and cognitive function over time. Five neurocognitive tests were predictive of improved speech perception following implantation. |
| Jayakody, et al. [51] | CI recipient (n=16) vs. CI candidate (n=23) | 61.8 (±15.6) vs. 69.0 (±12.4) | 34.4 (±18.6) years vs. 24.0 (±21.8) years | Assessments at the baseline (preop), 6 and 12 months postimplantation, by means of six subtests of the CANTAB: the attention switching, delayed matching to sample, paired associates learning, verbal recognition memory, reaction time, and spatial working memory task | Independent-sample t test scores for the changes between 0 and 12 months revealed that CI recipients performed significantly better on measures of simple reaction time, cognitive flexibility, paired-associate learning, working memory, and strategy use (p0.05) compared with implant candidates. Compared with the candidates, recipients also showed significantly lower stress scores (p0.05) after 1 year use of a CI. |
| Mosnier, et al. [47] | 94 (postlingual deafness) | 72 (±5.0) (range: 65-85) | 11 (±15.1) years | Evaluated before, 6 months after, and 12 months after CI using a battery of 6 tests evaluating attention, memory, orientation, executive function, mental flexibility, and fluency (MMSE, 5-word test, clock-drawing test, verbal fluency test, d2 test of attention, and TMT parts A and B) | The participants with preoperative abnormal scores showed significant improvements in all tests except in the CDT. The improvement was significant as early as six months for the MMSE (p=0.02), the FWT (p=0.004), the d2 test of attention (speed) (p=0.008), and the TMT part B (p=0.03), and became significant at 12 months for the TMT part A (p=0.02) and the number of errors on the d2 test of attention (p0.001). The participants with normal preoperative scores remained stable over time for most tests. A significant decline was observed, however, in performance on the FWT at 6 and 12 months (p=0.002). Also the CDT presented a significant decline at 12 months after implantation (p=0.046). |
| Sonnet, et al. [52] | 16 (postlingual deafness) | 72.5 (±5.3) (range: 65-80) | 17 years (mean duration of hearing aid use: 15 years) | Assessed prior to, at 6 and 12 months after implantation using cognitive test battery comprised the MMSE, the Rey complex figure test, TMT parts A and B, FWT, and test de dénomination orale d’image (DO80) | Overall, no significant changes were found in performance on any of these cognitive tests across the three measurements. |
| For instance, the mean MMSE score was 27.1 (±2.1), 26.0 (±3.0), and 27.7 (±1.6) prior to, at 6 and 12 months after surgery respectively. |
CI, cochlear implantation; CODEX, Cognitive Disorders Examination; MoCA, Montreal Cognitive Assessment; GDS, Geriatric Depression Scale; NH, normal hearing; WASI, Wechsler Abbreviated Scale of Intelligence; TMT, Trail Making Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; CANTAB, Cambridge Neuropsychological Test Automated Battery; MMSE, Mini-Mental State Examination; CDT, clock-drawing test; FWT, 5-word test




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download