Introduction
Obstructive sleep apnea (OSA) is a respiratory disorder characterized by repetitive upper airway obstruction, which leads to nocturnal hypoxia, hypercapnia, arousal during sleep, and associated sympathetic activation during sleep. These events cause various medical comorbidities, including diabetes mellitus, hypertension (HTN), and major cardiovascular events [
1]. Therefore, proper management of OSA is essential to prevent these complications. The treatment of OSA aims to maintain upper airway patency during sleep, and positive airway pressure (PAP) therapy is a first-line treatment modality for moderate-to-severe OSA as well as some mild OSA cases [
2]. It has been reported that PAP therapy not only improves quality of life but also reduces the risk of cardiovascular complications and sudden death in patients with OSA [
3-
5]. Although PAP therapy is an effective treatment modality for OSA, it has significant limitations in terms of adherence [
6]. Weaver and Grunstein [
7] reported that approximately 29% to 83% of OSA patients are non-adherent to PAP therapy. Therefore, it is very important to assess the factors related to PAP adherence and take appropriate treatment accordingly.
Since July 2018, the Korean National Health Insurance Service (NHIS) has been covering PAP therapy in patients with moderate-to-severe OSA (apnea-hypopnea index [AHI] ≥15) and some complicated patients (lowest SpO2 <85% or lowest SpO2 ≥85% with insomnia, daytime sleepiness, cognitive disorder, mood disorder, HTN, history of cardiovascular disease or history of cerebrovascular disease) in mild OSA (5≤AHI <15). The NHIS initially covers 80% of PAP device rental fee during the 3-month PAP device adaptation period and continues to provide the patients who use the device for ≥4 h/day and on 70% of 30 consecutive days with financial benefits; they pay only approximately 15 United States dollars per month for the PAP device rental fee. However, according to a recent announcement, the NHIS is considering to reduce the benefit for patients with an AHI value of 5 to 10 are under the assumption of reduced adherence to PAP therapy in these patients. Therefore, we conducted this study to compare the adherence to PAP therapy in patients with different OSA severity. Moreover, we compare the adherence to PAP therapy in patients with mild OSA by categorizing them according to their AHI values by 10 criteria.
Go to :

Results
Among 270 study subjects, 244 were male. The mean age of study subjects was 49.22±10.87 years. The mean and median values of the percentage of days on which subjects used the device of were 90.03±14.21% and 94.9% (85.7%-100%), respectively. The mean and median values of the percentage of days on which subjects used the device for ≥4 h were 80.13±20.61% and 85.7% (72.4%-95.2%), respectively. The mean and median values of the average duration of daily PAP device usage were 360.61±64.39 min and 362.50 min (324-400 min), respectively. In this study, 31, 68, and 171 subjects were diagnosed with mild, moderate, and severe OSA, respectively. The percentage of patients who were eligible for NHIS financial benefits in the mild, moderate, and severe OSA groups were approximately 83.9% (26/31), 88.3% (60/68), and 97.7% (167/171), respectively. We further divided 31 mild OSA patients into two categories according to their AHI values: AHI value 5-10 and AHI value 10-15 groups; 86.7% (13/15) patients in the AHI value 5-10 group and 81.3% (13/16) patients in the AHI value 10-15 group were eligible for NHIS financial benefits.
Table 1 describes patients’ overall demographic characteristics according to their OSA severity.
Table 1.
Demographic data of study subjects according to OSA severity
|
Mild OSA (n=31) |
Moderate OSA (n=68) |
Severe OSA (n=171) |
p value |
|
Age (years) |
46.32±8.84 (47 [40-52]) |
51.87±10.20 (54 [43-59]) |
48.70±11.29 (51 [40-57]) |
0.015*§
|
|
Sex (M:F) |
28:3 |
59:9 |
157:14 |
0.480‡
|
|
BMI (kg/m2) |
25.64±3.01 (25.7 [23.4-27.2]) |
26.30±2.72 (26.0 [24.5-28.1]) |
29.35±4.79 (28.5 [26.0-31.6]) |
<0.001*§
|
|
AHI (/hour) |
9.88±2.86 (10.1 [7.7-12.2]) |
21.18±4.31 (21.3 [17.1-25.1]) |
59.55±18.78 (60.8 [43.1-70.9]) |
<0.001*§
|
|
RDI (/hour) |
23.28±9.09 (23.0 [15.6-30.0]) |
31.90±8.58 (30.4 [26.1-35.5]) |
65.37±16.98 (64.4 [54.8-73.8]) |
<0.001*§
|
|
Arousal index (/hour) |
30.97±16.18 (28.4 [18.9-39.5]) |
34.80±11.27 (34.2 [25.9-40.3]) |
62.85±18.25 (62.1 [50.6-70.6]) |
<0.001*§
|
|
minSaO2 (%) |
78.82±12.28 (82 [75-86]) |
78.09±7.24 (79 [75-83]) |
66.29±10.85 (70 [61-77]) |
<0.001*§
|
|
ODI (/hour) |
8.12±2.89 (8.8 [5.5-10.3]) |
18.05±5.40 (18.0 [14.5-22.3]) |
55.14±20.48 (55.5 [39.6-67.3]) |
<0.001†§
|

The percentage of days on which patients used a PAP device during sleep did not differ significantly according to OSA severity in the mild, moderate, and severe OSA groups (
Table 2). There was also no difference in the percentage of days on which patients used a PAP device during sleep between the AHI value 5-10 and the AHI value 10-15 groups (AHI value 5-10 group: 94.30% [83.30%-100%] vs. AHI value 10-15 group: 88.55% [77.15%-96.63%],
p=0.313). We also found that the percentage of days on which a PAP device was used for ≥4 h during sleep did not differ significantly according to OSA severity; the percentage showed a tendency to increase with an increase in OSA severity (
Table 2). Moreover, in the subgroup analysis in the mild OSA group, the AHI value 10-15 group showed lower percentage of days on which a PAP device was used for ≥4 h than the AHI value 5-10 group although the difference was not statistically significant (AHI value 5-10 group: 83.90% [72.40%-91.30%] vs. AHI value 10-15 group: 74.05% [64.15%-85.25%],
p=0.115).
Table 2.
Adherence of PAP therapy as OSA severity
|
OSA severity |
Percentage of days on which patients used a PAP device |
p value |
Percentage of days on which a PAP device was used for ≥4 h |
p value |
|
Mild (n=31) |
91.40 [81.25-97.65] |
0.268 |
76.20 [69.70-89.90] |
0.097 |
|
Moderate (n=68) |
94.50 [86.90-100] |
|
89.30 [67.65-95.70] |
|
|
Severe (n=171) |
95.90 [88.10-100] |
|
85.70 [75.85-95.45] |
|
|
5≤AHI<10 (n=15) |
94.30 [83.30-100] |
0.313 |
83.90 [72.40-91.30] |
0.115 |
|
10≤AHI<15 (n=16) |
88.55 [77.15-96.63] |
|
74.05 [64.15-85.25] |
|
|
Moderate (n=68) |
94.50 [86.90-100] |
|
89.30 [67.65-95.70] |
|
|
Severe (n=171) |
95.90 [88.10-100] |
|
85.70 [75.85-95.45] |
|

We determined the adequate adherence to PAP therapy in patients according to the Centers for Medicare and Medical Services guidelines by using a PAP device for ≥4 h/day and during ≥70% of nights [
6]. We found that the percentages of patients with adequate adherence to PAP did not differ significantly according to OSA severity (mild: 74.2%, moderate: 72.1%, severe: 83.0%,
p=0.084). In addition, the percentage of patients with adequate adherence to PAP did not show significant difference between the AHI value 5-10 and the AHI value 10-15 groups (AHI value 5-10 group: 86.7% vs. AHI value 10-15 group: 62.5%,
p=0.245) (
Fig. 2).
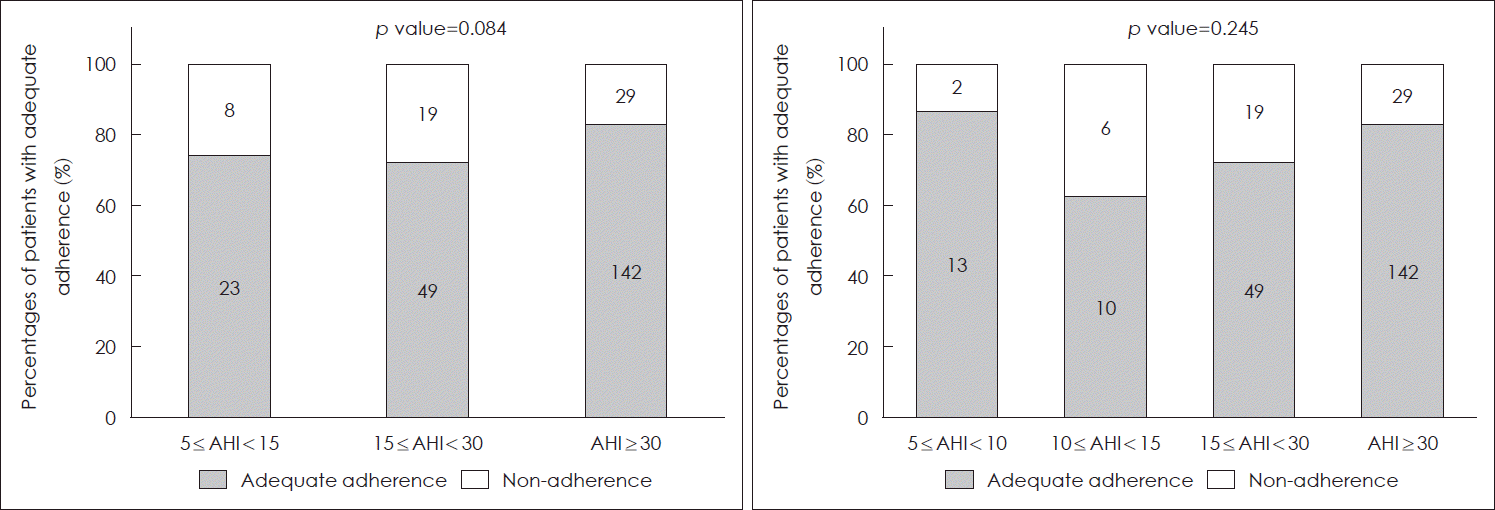 | Fig. 2.Percentages of patients with adequate adherence to positive airway pressure therapy in the mild, moderate, and severe obstructive sleep apnea groups. Fisher’s exact test. AHI, apnea-hypopnea index. 
|
The mean duration (minutes) of PAP device usage during sleep did not differ significantly according to OSA severity (mild: 348.03±47.78 min, moderate: 358.58±85.22 min, severe: 363.79±57.21 min,
p=0.440). It also showed no difference between the AHI value 5-10 and the AHI value 10-15 groups (AHI value 5-10 group: 361.53±33.19 min vs. AHI value 10-15 group: 335.38±56.44 min,
p=0.405) (
Fig. 3).
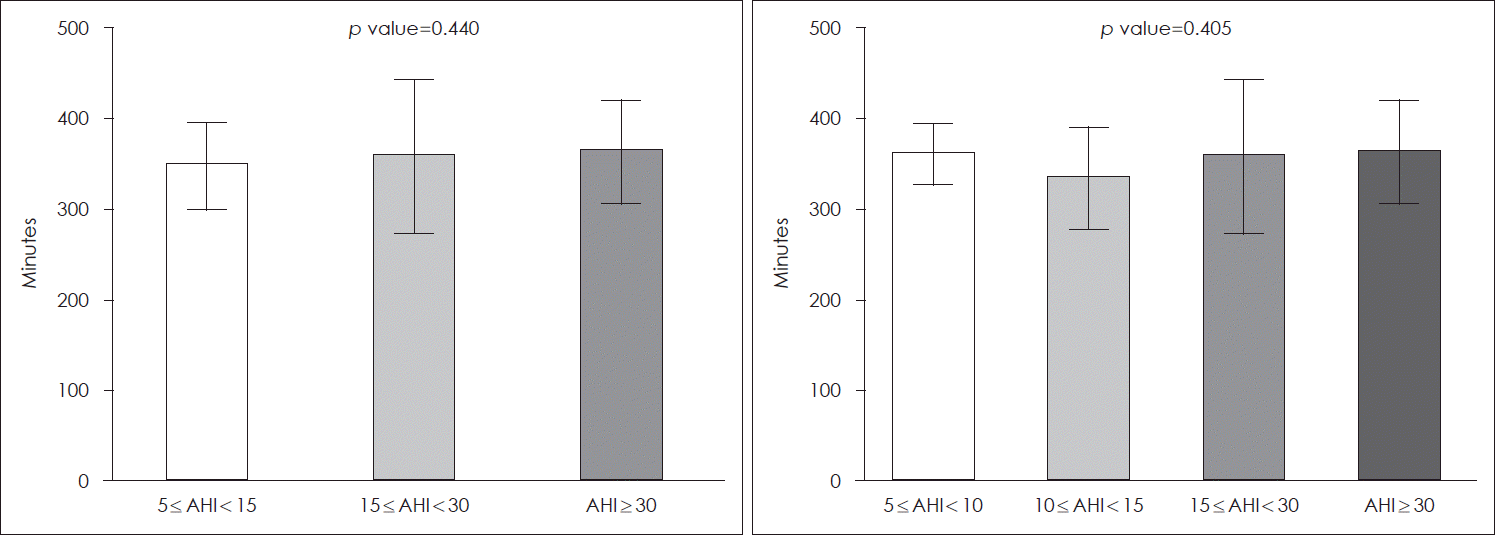 | Fig. 3.Mean duration of positive airway pressure device usage during sleep in patients with different obstructive sleep apnea severity. One-way analysis of variance with Bonferroni correction. AHI, apnea-hypopnea index. 
|
Go to :

Discussion
In the present study, there were no significant differences in various parameters regarding adherence to PAP therapy (i.e., the percentage of days on which the device was used, the percentage of days on which the device was used for ≥4 h, and the average duration of daily PAP device usage) among patients with different OSA severity. To date, many studies have been conducted to elucidate the factors related to adherence to PAP therapy [
6]. In particular, although many studies have reported OSA severity as one of the factors that affect adherence to PAP therapy, their results differ. Riachy, et al. [
10] performed a cross-sectional study with 138 OSA patients and reported that the mean respiratory disturbance index and oxygen desaturation index were significantly higher, and the minimum SaO
2 value during sleep was significantly lower in the adequate adherent group at 3 months after PAP therapy than non-adherent group. In addition, Campos-Rodriguez, et al. [
11] performed a cross-sectional study with 357 OSA patients and reported that AHI and the percentage of duration with oxygen desaturation of <90% were significantly higher in the adequate adherent group than non-adherent group. Moreover, according to a meta-analysis by Madbouly, et al. [
12], AHI was significantly higher in the adequate adherent group than in the non-adherent group. Therefore, many physicians believe that the severity of OSA might significantly affect adherence to PAP therapy. In addition, Baratta, et al. [
13] recently performed a cross-sectional study with 295 OSA patients and reported that a low OSA severity was an independent predictor of nonadherence to PAP therapy. However, that study was conducted only in patients with moderate-to-severe OSA (AHI ≥15). In addition, our subjects were motivated to use a PAP device for financial benefits from the NHIS during the study period, and there might be some differences in results from other studies. We conclude that the severity of OSA does not affect adherence to PAP therapy in OSA patients. Therefore, physicians should not hesitate to treat mild OSA patients with PAP therapy due to non-adherence to PAP therapy in those patients in South Korea.
To the best of our knowledge, this is the first study that assessed PAP adherence in patients with different OSA severity, including patients with mild OSA, and determined various parameters regarding PAP adherence in mild OSA patients by subdividing them according to their AHI values (5-10 vs. 10-15). In this study, the mild OSA group showed the lowest percentage of days on which subjects used a PAP device for ≥4 h during sleep. Although this observation was not statistically significant, it might indicate that PAP adherence is poor in the mild OSA group. However, in the subgroup analysis in the mild OSA group, the median value of the percentage of days on which a PAP device was used for ≥4 h during sleep was lower in the AHI value 10-15 group than in the other groups; the difference was not statistically significant. Furthermore, we found that the median value of the percentage of days on which a PAP device was used for ≥4 h during sleep was lower in the AHI value 5-10 group (83.90% vs. 85.70%) than in the severe OSA group. Therefore, we believe that this tendency was due to the results observed in AHI 10-15 group, and adherence to PAP therapy in the AHI value 5-10 group was not inferior to that in the severe OSA group.
One of the well-known factors related to PAP therapy adherence is patients’ socioeconomic status (SES). Bakker, et al. [
14] performed an observational study with 126 patients and reported high individual socioeconomic deprivation as a significant independent predictor of poor CPAP adherence. In addition, Simon-Tuval, et al. [
15] reported that patients with low SES are less receptive to CPAP treatment than those with higher SES. Therefore, financial burden of PAP therapy might be considered as an independent factor that negatively affects the continuation of PAP therapy. In this study, the difference in PAP therapy adherence according to OSA severity was not significant because financial benefits were provided equally. As mentioned above, the NHIS in South Korea initially covers 80% of the PAP device rental fee during the 3-month PAP device adaptation period and continues to provide financial benefits to the patients who use the device for ≥4 h/day and on 70% of 30 consecutive days; patients need to pay only approximately 15 United States dollars per month for renting the device. A reduction in financial benefits in this situation will result in a decrease in PAP adherence, and it will not be an appropriate measure in terms of reduction in patient morbidity.
Several studies reported that the percentage of patients with adequate adherence to PAP therapy at 3 months after initiation of PAP therapy was about 50 to 60% [
8,
16]. In addition, the percentage of nights during which the device was used for at least 4 h at 3 months after initiation of PAP therapy was reported as 49±36% or 63±29% according to titration protocol [
8]. In our study, the percentage of patients with adequate adherence to PAP therapy was 79.3% (216/270), and the percentage of nights during which the device was used for at least 4 h was 80.13±20.61% at 3 months after initiation of PAP therapy. Moreover, a previous study by Kim, et al. [
17], which was performed in South Korea before the Korean NHIS started to cover PAP therapy, reported that only 34.7% of patients used PAP device for more than 3 months although their methodology was not exactly the same as that of the present study. We believe that the primary reason for adherence to PAP therapy observed in our study being higher than that observed in previous studies might be due to the alleviation of economic burden by insurance coverage. Therefore, we could confirm once again that reduction in financial burden plays an important role in increasing PAP device adherence.
This study has some limitations. First, it was a retrospective medical record review study; therefore, the authors might not control some confounding factors that affect the results of this study. However, although this study was conducted retrospectively, it included a sufficient number of subjects, and the data was collected intentionally and prospectively. Therefore, we regarded that our result is meaningful as prospective study. Second, the majority of participants were male. Thus, the results of the present study might reflect a selection bias. However, OSA is more prevalent in men than in women because of its nature. Third, the median age of the present study was significantly different among each groups. Therefore, it might also reflect a selection bias and this should be taken account in the interpretation of the results of the present study. Forth, as this study is a retrospective study, it does not include a control group that did not receive financial support. Therefore, our results do not sufficiently reflect this factor. Further research is needed to determine whether our conclusion remains adequate in the long term.
In conclusion, PAP therapy adherence did not differ according to OSA severity in adult patients. Moreover, PAP therapy adherence in the AHI value 5-10 group was not inferior to that in the moderate or severe OSA group. Compared with previous studies, adherence to PAP therapy is very high within the current national insurance system of South Korea. In particular, since there is no difference in PAP adherence according to the severity determined by AHI, aggressive PAP therapy might be considered in patients with mild OSA. This high degree of compliance may have a great influence on the support provided by the national insurance for the prescription of PAP devices. Therefore, it is necessary to continuously expand and maintain the insurance for the health promotion in OSA patient in South Korea.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download