Introduction
Nasal irrigation (NI) plays a vital role in pre-and post-endoscopic sinus surgery (ESS) to treat chronic rhinosinusitis (CRS). Moreover, a Cochrane review has shown that NI plays a role in improving symptoms and the quality of life (QOL) [
1]. NI serves as a non-invasive adjunctive postoperative treatment that reduces the antigens and biofilm and provides mucociliary clearance in the sinonasal mucosa [
2]. Clinically, various irrigation methods have been used for physical cleaning and mucosal healing during the postoperative period after endoscopic sinus surgery.
Most studies on postoperative NI have been about saline composition or adjuvants using the classic manual bottle-squeeze method [
3-
5]. To date, normal saline solutions remain the standard formulation, with no significant difference between different solutions based on salt concentration [
6]. A recent cadaveric study comparing a pulsating device and the bottle-squeeze method for sinus penetration of the irrigant has concluded that the manual method is superior in native and operated conditions [
7]. However, to the best of our knowledge, a paucity of information exists regarding the efficacy of powered irrigation devices in living patients after endoscopic sinus surgery. Moreover, there are several commercially available devices for postoperative NI. However, there is also little information in the literature verified by the systematic comparative trials.
This study compared a novel automatic device with the traditional manual method in an actual clinical setting. We compared the clinical distinction and patients’ perspectives between the two methods at various postoperative periods using subjective questionnaires and postoperative endoscopic findings. We aimed to compare the efficacy of the powered and manual irrigation methods in postoperative care and validate the convenience based on patient perspectives.
Subjects and Methods
Patients and study design
This prospective randomized single-blinded study was conducted in a clinical trial setting. The inclusion criteria were: 1) adult patient (≥20 years old) diagnosed with CRS; 2) refractory to optimal medical therapy and required endoscopic sinus surgery; 3) no history of sinonasal surgery. The exclusion criteria were: patients with 1) incomplete clinical data; 2) pregnancy or breastfeeding; 3) neoplasm; 4) uncontrolled systemic comorbidities; and 5) participation in other clinical trials within a month.
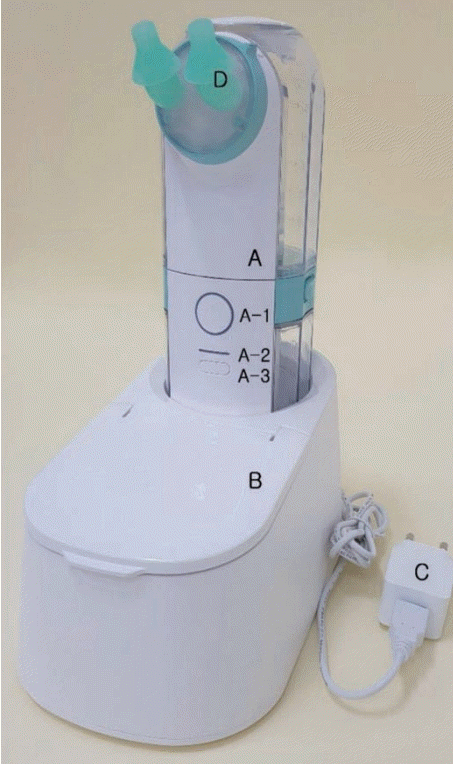
A total of 29 patients were included in the study and divided into two groups: 1) the NOSSHA
® group (NI using a powered irrigation device, n=14), and 2) the control group (manual bottle-squeeze irrigation, n=15). The grouping was done by stratified randomization sampling. NOSSHA
® (Womens Care Co., Ltd., Seoul, Korea) is a recently developed novel automatic sinonasal wash system that provides a constant volume and pressure, irrespective of the effort applied by the patient (
Fig. 1). NOSSHA
® is one of the electrically operated high-volume and high-pressure devices. The device comprises the upper tank, the storage cradle, the nozzle in close contact with nostrils, and the washing tube. The maximum irrigation fluid volume is 250 mL which is the same as the manual bottle. The intensity of the irrigation pressure is controlled in 3 steps by pressing the button located in the center. The device is connected to the charging cradle for storage, and washing or sterilization is possible after separating the upper tank, the lower tank, and the nozzle. On the other hand, the control method– manual bottle-squeeze irrigation-is high-volume and low-pressure. The volume is the same in the NOSSHA
® and control groups.
This study was approved by the Institutional Review Board of Konyang University Hospital (KYUH 2018-11-005-002). All participants provided written informed consent.
Surgery and posr-operative management
The ESS was performed under general anesthesia by a single surgeon in our hospital. All surgical procedures were performed using the standard technique for ESS, as previously reported [
8], and no iatrogenic complications were encountered. The wound was packed with the absorbable nasal dressing, Nasopore
® (Polyganics, Groningen, The Netherlands), and broad-spectrum oral antibiotics and mucolytics were prescribed in all patients. The absorbable nasal dressing was removed endoscopically at 2 days postoperatively; systemic corticosteroid was not prescribed while topical corticosteroid spray was used for all patients until 2 months after packing removal.
After the surgery, the patients in the NOSSHA® group used the powered irrigation device twice daily, while the control group used the manual bottle twice daily. We assessed the patients’ status at baseline and 1, 2, 4, 6, and 8 weeks postoperatively, using each item in the questionnaires in the outpatient office.
Outcome measurements
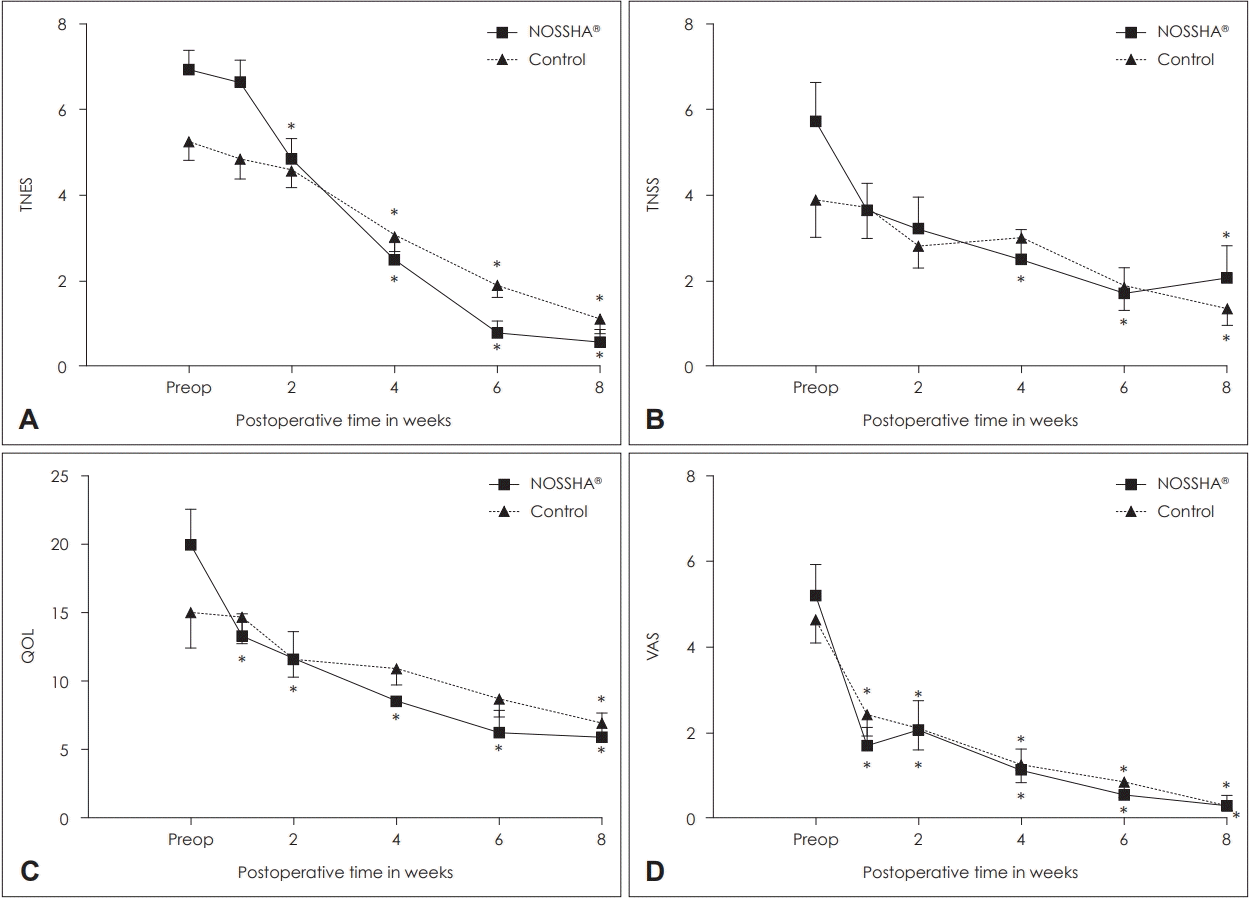
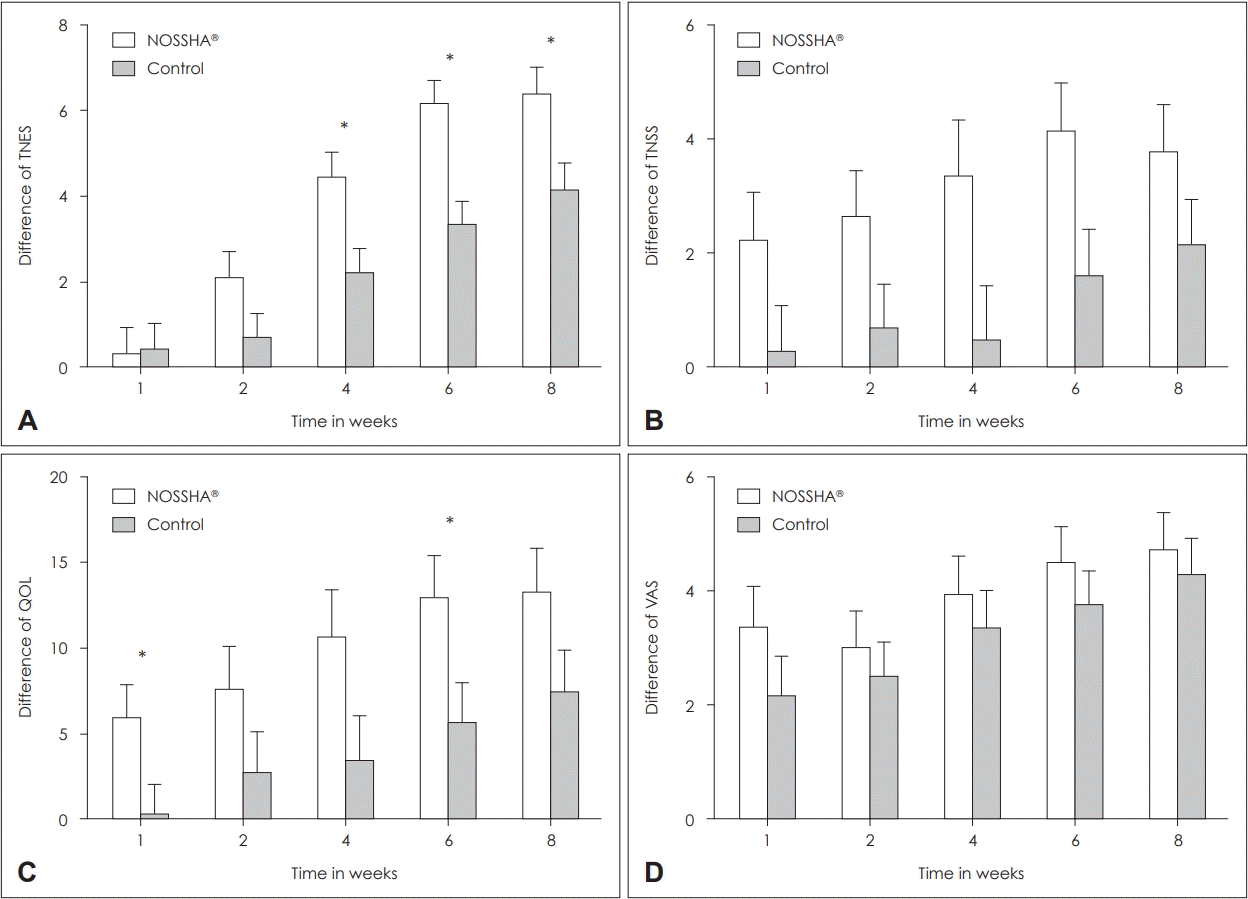
The participants underwent evaluations that included the total nasal endoscopic score (TNES), total nasal symptom score (TNSS), visual analog scale (VAS) score, and QOL questionnaires. The TNES was evaluated using nasal endoscopy (modified Lund-Kennedy score, as previously described) [
9]. The TNES was checked by two experienced otolaryngologists blinded to the grouping independently. Additionally, the patients also completed three patient-reported outcome measures, including TNSS, QOL, and VAS. The TNSS consisted of rhinorrhea, nasal congestion, nasal itching, and sneezing. Each group was evaluated using 0-3 points, with a maximum of 12 points, and each group was analyzed at all points [
10]. The QOL questionnaire evaluated the areas of a patients’ QOL that were affected by rhinosinusitis. It consisted of five questions that allotted scores to rhinology symptoms caused by stimuli [
11]. The VAS is a reliable indicator of pain and was used to assess pain intensity after NI. The participants rated pain on a scale of 0 (no pain) to 10 (worst or intolerable pain) [
12].
Statistical analysis
General statistics are presented as mean±standard error of the mean for continuous data and frequency (%) for categorical data. The baseline characteristics of the study participants were compared between the NOSSHA® and control group using the independent t-test, (interquartile range [Q1, Q3]) or Pearson’s chi-square test, as appropriate. The outcome and the difference in the scores from the baseline were assessed using the mixed effect model for repeated-measures (MMRM) analysis with an unstructured covariance matrix. Furthermore, in the MMRM analysis, the differences in the baseline scores were also compared between the groups after adjusting for age, sex, and corresponding baseline score. All statistical analyses were conducted using IBM SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA) and GraphPad Prism Software Version 5.02 (GraphPad Software, San Diego, CA, USA). Statistical significance was declared in two-sided tests at a significance level of ≤0.05.
Discussion
NI has been recommended as an additional non-pharmacological therapy for patients with CRS and may be considered a first-line treatment for this condition [
13]. Moreover, based on a 2016 Cochrane review study [
14], NI has been strongly recommended as a potentially beneficial low-risk treatment option for CRS. Additionally, its application may have an indubitable role in the postoperative healing process [
15]. A wide variety of irrigation methods have been investigated for clinical applications, including their components, hygiene status, and mode of delivery [
16]. However, there is insufficient clarity regarding the tonicity and adjunctive solutions used in NI [
17,
18]. Moreover, the frequency and duration of NI in the literature are controversial [
2,
13].
However, regarding physical factors, the high-volume and low-pressure methods have been determined as the standard for sinonasal irrigant delivery. Typical high-volume and low-pressure delivery methods include manual squeeze bottles and gravity-dependent irrigation pots. One systematic review recommends high-volume devices for optimum paranasal sinus irrigant delivery after ESS [
19]. They recommend using the lying-head-back or lateral head-low position with low-volume devices for patients who are intolerant to high-volume devices.
Recent cadaveric studies have validated the influence of surgical method and delivery position of irrigant on sinus penetration [
20,
21]. Although high-volume and low-pressure devices are optimal for postoperative use, possible disadvantages NI using these devices include bothersome effort, uncomfortable sensation, burning, and Eustachian tube irritation [
19]. Low-volume devices, such as spray and nebulizers or high-pressure powered devices, may be alternatives for these patients.
Piromchai, et al. [
22] have reported that low-volume and high-pressure devices received significantly higher physicians’ and patients’ scores in a Thai multicenter survey. They also stated ease of learning as an advantage and retained fluid in the sinuses as a disadvantage.
In this study, a significant improvement was observed endoscopically, which was achieved earlier with the high-pressure powered device than with the manual bottle-squeeze method. Interestingly, this observation became more pronounced toward the end of the follow-up period. The powered irrigation system ensures consistent pressure, which is impossible with the manual squeeze method by living patients. Based on this finding, we assert that constant-pressure irrigation may provide greater mucosal healing over a late period than during the immediate postoperative period. From the patient’s perspective, an improvement in the rhinology symptoms (TNSS) and pain (VAS) was not significantly different between the two groups. In contrast, the difference in the QOL was superior to the powered irrigation system. Additionally, through the TNSS and QOL measures, symptomatic improvement achieved with the use of the powered device was observed to be earlier than that with the manual bottle method.
The manual bottle-squeeze method requires unnecessary effort from the patients, whereas a powered irrigation device may be more convenient. In addition, by providing constant volume and pressure, a powered device can prevent eustachian tube irritation caused by applying unintended pressure. The irrigation device may be adjusted according to user convenience at different stages with a constant flow rate and pressure. This finding implies that the powered irrigation system may become an alternative therapeutic option for patient compliance.
There are similar NI devices that could be searched online. However, we could not find any evidence-based literature regarding these commercially available products. In the present study, the authors evaluated comparative clinical trials on powered NI and the golden standard treatment–the manual irrigation, based on subjective symptoms and endoscopic findings according to the postoperative periods. To the best of our knowledge, this is the first English article that tried to verify the clinical use of powered NI.
Regarding the side effects of this powered irrigation device, there was no evidence of iatrogenic otitis media due to the reflux from the eustachian tube or infections associated with nasal irrigation. However, there was a technical error at the outlet grounding part. The electrical short-circuit due to poor contact in the ground part of the power outlet was reported. There was no particular risk to the patient because the undesirable problem was at the electrical grounding area, not the area used by patients. The ground part should be plated with certainty, such as gold or silver, due to the electrical conductivity with saline solution. The device is not available for clinical use yet, but only for the research purpose at this time for technical reason. Further work would be needed for the technical correction because the device’s safety has not been fully verified.
There are also some methodological limitations of this study. First, our study was limited by small sample size (approximately 15 patients per group). Second, there was a score gap between the two groups at the baseline. However, we adjusted this baseline discrepancy using the MMRM statistical method. The MMRM method can handle baselines after randomization in the clinical comparative study like this study [
23]. Third, we evaluated the patients for a relatively short-term followup period (8 weeks). In contrast, the wound healing process in the sinonasal epithelium is usually completed within 11-14 weeks after ESS [
24]. Fourth, we did not evaluate the patient’s compliance. A future study using a larger number of patients with a long-term follow-up is needed to expand scientific knowledge and overcome the limitations of this study.
There are several strengths regarding the powered NI method. First, endoscopically, powered NI can provide a constant pressure, which is controlled in 3 steps. This helps faster and proper mucosal healing, particularly in the late postoperative period than manual bottle irrigation. Second, otologically, powered NI can avoid the iatrogenic otalgia or middle ear effusion from too forceful strength of irrigation by manual method. Third, subjectively, the electronic device provides clean and comfortable environments. The sterilization, soft nozzle to nostrils, without sink space for drain out, and without lying-head-down for irrigation would be another strength for powered NI device. In terms of drawbacks, the size of electronic devices is relatively larger than a classic bottle due to a power cord, operating tank, or irrigation tube. This could be a potential drawback for patients who travel often. In addition, the cost of electronic devices would be expensive than that of a plastic bottle. It may be five times more expensive than a classic bottle estimated online by similar products (approximately USD $100 vs. USD $20). The exact comparison could not be determined because NOSSHA® is not approved for clinical use yet.
In conclusion, NI with constant pressure provides rapid postoperative improvement. Using a powered device for NI may be suitable for patients who are unable to tolerate manual irrigation as an alternative therapeutic option. In addition, further research would be needed to verify the safety of electronic nasal irrigation technically and also clinically.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download