Abstract
Background and Objectives
The objectives of this study were to investigate the aspiration patterns in patients with T3 and T4 oral and oropharyngeal cancers after free flap reconstruction following primary tumor resection and determine the effect of tongue base resection on aspiration patterns in these patients.
Subjects and Method
The aspiration pattern was evaluated via fiberoptic endoscopic evaluation of swallowing and classified into three groups based on the timing of aspiration in relation to the swallowing process. More than two types of aspiration patterns observed simultaneously in a patient suggested combined aspiration pattern.
Results
The major pattern of aspiration in 31 patients with oral cavity cancer was aspiration after swallowing in the group with base of tongue (BOT) preserved (83.3%, 10/12) and combined aspiration in the group with BOT resection (63.2%, 12/19), showing a significant difference in aspiration pattern between the two groups (p<0.001). In oropharyngeal malignancies, the major pattern of aspiration was aspiration after swallowing in both BOT-preserving (87.5%, 21/24) and BOT-resecting groups (75.0%, 9/12), showing a significant difference in aspiration pattern between the two groups (p=0.031).
Safe and effective swallowing can be achieved by harmonious and complex movements of structures located in the head and neck. Although it is difficult to simplify the swallowing process, it can be divided into preparatory, oral, pharyngeal, and esophageal phases, with each phase having its importance [1]. Treatment for head and neck cancer (HNC) can impair the structure and function of components involved in oral and pharyngeal phases, resulting in dysphagia or aspiration, especially in patients with advanced-stage disease [2,3]. Aspiration in HNC is a frequent and annoying challenge that causes significant malnutrition and degrades the quality of life of patients. Therefore, postoperative evaluation and management of aspiration are of great importance [4,5].
Head and neck structures play important roles in each stage of the swallowing process for an effective swallowing [1,2]. Tongue, the structure where cancer is most prevalent in the oral cavity, plays an important role in the control of bolus in the oral stage. It triggers the pharyngeal phase by providing sensory information about the bolus [6,7]. The pressure generated by the pharyngeal wall and the base of tongue (BOT) followed by laryngeal closure plays an important role in the esophageal stage without aspiration after a successful transition of bolus into the pharynx [7]. Since major surgeries for oral and oropharyngeal cancers eliminate at least one of the structures associated with swallowing, the function of bolus control in the oral stage or the generation of pressure in the pharyngeal stage may be impaired, especially for those in advanced T stage [4,8]. Simultaneous resection of surrounding normal tissues along with the primary tumor is needed to ensure a sufficient surgical margin. BOT contributes to both bolus control in the oral cavity and pressure generation in the pharynx. It is frequently resected along with the primary tumor in the oral cavity and oropharynx for achieving an adequate surgical margin in advanced oral and oropharyngeal cancers [4,9]. Although a large surgical defect is generally reconstructed by a free flap, impaired physiology of swallowing due to surgery is often not fully restored in these patients due to bulky flap and immobility [10,11]. The aspiration pattern observed in fiberoptic endoscopic evaluation of swallowing (FEES) differs according to the impaired swallowing mechanism. FEES provides information about the impaired swallowing mechanism that needs to be corrected to ensure successful oral intake postoperatively [12,13]. Since FEES is conducted under a laryngoscopic view which is familiar to head and neck surgeons, it allows surgeons to directly understand the causes of aspiration and evaluate the effectiveness of swallowing exercise and maneuver after the surgery [14,15].
We postulated that aspiration pattern could be determined based on the primary site of cancer after tongue and oropharyngeal cancer surgery and that BOT resection could cause more complex swallowing impairment than a simple estimation based on the inherent function of primary site during FEES despite a flap reconstruction. Thus, the objectives of this study were: 1) to investigate aspiration patterns in patients with T3/T4 oral and oropharyngeal cancers after free flap reconstruction following primary tumor resection; and 2) to determine the effect of BOT resection on aspiration patterns in these patients.
This retrospective study was approved by the Institutional Review Board of our institution (IRB number: 14-2-21). Consecutive patients who underwent curative surgeries for T3-T4 oral and oropharyngeal cancer via free flap reconstruction at a single referral hospital from September 2010 to December 2013 were reviewed. The type of free flap used for reconstruction was decided arbitrarily by an experienced reconstructive surgeon based on the location and the extent of the surgical defect. Tongue base resection was defined when tongue base resection of greater than 25% [16]. Patients with a history of aspiration pneumonia, cerebrovascular diseases, irradiation of head and neck, neuromuscular degenerative diseases, esophageal disease, or previous head and neck surgery that might affect swallowing were excluded from this study. Patients with complaints of poor oral intake or aspiration at the initial presentation were also excluded.
FEES was conducted during the period between surgical wound stabilization and adjuvant therapy (radiation or chemoradiation). The duration of initial FEES for inclusion was less than 90 days after surgery in this study. All FEES examinations were performed by a single experienced head and neck surgeon. All patients were examined in an outpatient setting while sitting in an upright position. A flexible fiberoptic laryngoscope (ENF-P4; Olympus Optical Co., Tokyo, Japan) was inserted through the nasal cavity without local anesthesia. Assessments for anatomical changes and dynamic movements of the oropharynx, hypopharynx, and larynx caused by surgery were similar to those of regular laryngoscopy. A bolus was loaded into the oral cavity from a syringe or a spoon after the tip of the endoscope was positioned between the soft palate and theepiglottis showing the entire BOT, larynx, and hypopharynx. A 3 cc of semi-solid bolus was the initial choice for FEES evaluation for all patients. The bolus was increased to 5 cc and 10 cc for tolerant patients. The viscosity and the volume were adjusted based on the aspiration detected during FEES. Any premature spillage defined as bolus transition from the oral cavity to the vallecular or hypopharynx before swallowing initiation was monitored after bolus loading. In addition, the presence of residual bolus in the oral cavity after swallowing was assessed by an assistant via oral cavity inspection. Any inappropriate laryngeal closure was evaluated after initiating the swallowing. Consequent aspiration into the vocal fold was assessed in the immediate phase after ‘white-out.’ If ‘white-out’ was inadequate, pharyngeal contractions and posterior movements of BOT were then evaluated after repositioning the endoscope tip. A bolus residue in the vallecula or pyriform sinuses and its aspiration after swallowing were evaluated. Aspiration was determined when the material entered the airway, passed below vocal folds, and ejected into the larynx or out of the airway (penetration-aspiration scale≥6) [17]. All procedures were recorded as video files and blindly reviewed by two other experienced otolaryngologists.
Aspiration patterns were determined based on the timing of bolus aspiration as follows: 1) aspiration after swallowing, delayed aspiration due to bolus residue after swallowing (Fig. 1A); 2) aspiration during swallowing, impaired laryngeal closure; and 3) aspiration before swallowing, premature spillage of bolus before initiation of the pharyngeal phase (Fig. 1B) [13]. If more than two types of aspiration were observed in a patient, a combined type of aspiration was considered.
Statistical analyses of aspiration patterns depending on the specific structure were performed using independent t-test, Pearson’s chi-square test, and Fisher’s exact test using SPSS software version 21.0 (IBM Corp., Armonk, NY, USA). A p-value of less than 0.05 indicated statistical significance.
A total of 67 patients who underwent curative surgery along with free flap reconstruction for T3 or T4 tongue or oropharyngeal cancer according to American Joint Committee on Cancer 8th edition were included in this study. These eligible patients 31 (46.3%) cases of tongue cancer (24 males and 7 females) and 36 (53.7%) cases of oropharyngeal cancer (28 male and 8 female). The mean age of included patients was 61.6 (SD, 6.2) years. The mean duration between surgery and initial FEES examination was 25.9 (SD, 7.5) days. There was no significant difference in mean age (p=0.150), gender distribution (p=0.972), or mean duration from surgery to FEES (p=0.321) between the group with tongue and the groups with oropharyngeal cancer. All included patients underwent simultaneous neck dissection along with primary tumor resection because of their advanced stage of the disease. Types of surgery according to primary sites and reconstruction are shown in Table 1. Aspiration was found in 97.0% (65/67) of patients.
The primary site of malignancy was tongue in all 31 patients with oral cavity cancer, and no patient exhibited normal swallowing on FEES (Table 2). The major aspiration pattern was the combined type of aspiration (aspiration before swallowing and aspiration after swallowing) (64.5%, 20/31). Other patients experienced aspiration after swallowing (35.5%, 11/31). In patients with the combined type of aspiration, premature leakage of a bolus into the pharynx before pharyngeal phase initiation was identified. It was aspirated into the trachea before laryngeal closure (Fig. 1A). Subsequent transition of oral residue into the pharynx and pharyngeal bolus residue after swallowing caused aspiration. When patients were categorized into two surgical groups depending on whether or not BOT was resected, 12 (38.7%) of 31 patients showed BOT preservation. The majority (83.3%, 10/12) of these 12 patients reported aspiration after swallowing. Most patients who underwent simultaneous BOT resection with tongue cancer experienced the combined type of aspiration (aspiration before swallowing and after swallowing) (63.2%, 12/19). There was a significant difference in aspiration between the two groups (p<0.001) (Fig. 2A).
In oropharyngeal malignancies, the primary site was tonsil in 34 patients and BOT in 2 patients (Table 2). Ipsilateral oropharyngectomy including tonsil and contralateral tongue base resection was required for two patients with BOT cancer. Normal swallowing was observed in only 5.6% (2/36) of patients. On FEES, the circumferential contraction of the reconstructed site was reduced, and the pharyngeal residue was observed after swallowing was observed, which caused aspiration. The aspiration pattern occurred after swallowing in the majority of them (94.4%, 34/36). This pattern was also dominant in both BOT preservation (87.5%, 21/24) and resection groups (75.0%, 9/12). Differences in aspiration pattern were observed between patients with and without BOT preservation (p=0.031) (Fig. 2B). Combined aspiration pattern was observed in four patients including one patient with preserved BOT and three patients with resected BOT. Three of these four patients underwent resection of unilateral hypoglossal nerve due to suspected tumor invasion.
Normal swallowing can be achieved by successfully completing main tasks in each phase of the swallowing process. Impairment to a single stage of the swallowing process can lead to residue formation as a result of aspiration [1,2]. Since HNC surgery eliminates at least one structures critically involved in the swallowing process, impaired swallowing mechanism cannot be fully restored even after the defect is reconstructed via flap transfer, especially in those with an advanced T stage [3]. The rate of aspiration after T3/T4 oral and oropharyngeal cancer in this study was 97.0%. Thus, swallowing function should be evaluated and managed for these patients.
The impaired mechanism of swallowing caused by HNC surgery, which affects the aspiration pattern observed by FEES is somewhat predictable since structures with inherent swallowing functions are eliminated during surgery [4,10]. Since a volume loss of mobile tongue can lead to impaired bolus control in the oral cavity, any premature spillage of a bolus into the pharynx before pharyngeal phase initiation or immediate transition of the residue from the oral cavity into the pharynx after swallowing can result in aspiration [12]. Therefore, we postulate that aspiration before swallowing is a major pattern of aspiration because tongue is responsible for bolus holding in the oral cavity. In this study, all patients with T3/T4 tongue cancer showed aspiration after surgery despite they had free flap reconstruction. In addition, they predominantly showed the combined type of aspiration (aspiration before swallowing and aspiration after swallowing) [18,19]. Since occlusion of the oropharynx by forceful displacement of BOT is one of the important mechanisms generating pharyngeal pressure, the force of bolus propulsion might be attenuated after BOT resection, resulting in residual bolus and subsequent aspiration after swallowing even in patients with tongue cancer [19]. Interestingly, aspiration after swallowing was predominant in patients undergoing glossectomy in BOT preserved patients. A previous study has reported that glossectomy (partial or total) can degrade bolus control of the oral cavity, the upper esophagyeal sphincter relaxation, and the airway protection, resulting in aspiration before, during, and after swallowing [20]. Subsequent transition of oral residue into the pharynx and pharyngeal residue after swallowing observed in this study are important causes of aspiration after swallowing. The reason for not observing an isolated aspiration before swallowing is that extensive resection of tongue which impairs oral bolus control may be accompanied by other function impairments that might cause both oral and pharyngeal residue formations. Thus, glossectomy could impair the pharyngeal phase of swallowing even in patients with preserved BOT. Not only impaired bolus control in the oral cavity but also insufficient pharyngeal pressure due to BOT surgery can trigger aspiration after surgery for tongue cancer. Thus, rehabilitation to enhance pharyngeal pressure in addition to rehabilitation for bolus control in the oral cavity is necessary in patients undergoing major surgeries for tongue cancer, especially in patients with simultaneous BOT resection.
The contraction of pharyngeal wall during the pharyngeal phase of swallowing is essential to generate sufficient propulsion of the bolus into the esophagus [2]. A wide pharyngeal resection can weakens the pharyngeal wall contraction, resulting in residual bolus formation after swallowing followed by aspiration [6,21]. The proportion of those with aspiration after oropharyngeal surgery in advanced T stage was found to be extremely high. Aspiration after swallowing was found to be the main aspiration pattern in patients who underwent wide resection of oropharynx despite flap reconstruction in this study. Interestingly, the aspiration pattern differed when BOT was resected along with oropharyngeal cancer. The combined type of aspiration (aspiration after swallowing and aspiration before swallowing) in patients with BOT resection (3/12) was more frequent than in patients without BOT resection. Although BOT plays a great role in pressure generation in the pharyngeal phase, the mobility of the tongue is also significantly impaired when the extent of BOT resection is large. In addition, simultaneous scarification of hypoglossal nerve can cause severe degradation of tongue mobility [22]. These results suggest that a weak pharyngeal pressure is the main mechanism of aspiration after a wide resection of the oropharynx, and that bolus control can also be significantly impaired when BOT is resected.
This study has some limitations. Thus, caution is needed when interpreting the results of this study. First, although we used the classification widely accepted for determining aspiration pattern observed in FEES, there was a possibility that aspiration during swallowing might not be clearly detected. Pharyngeal contraction during swallowing inevitably causes “white out” when performing laryngoscopy. We had limited laryngeal observation due to bulky flap because FEES was conducted within three months after surgery. Thus, based on the results of this study, it was only possible to conclude that tongue base resection could cause a combined type of aspiration due to impairments of various swallowing mechanisms. Second, data were reviewed the data retrospectively. Therefore, patients who did not complain about dysphagia or were reluctant to undergo FEES might have been excluded, which might have resulted in a higher than the actual rates of aspiration. Third, the number of included patients was too small to provide a detailed analysis of aspiration pattern according to surgical procedures. Fourth, we described only aspiration patterns occurring in the pharynx after various types of tongue and oropharyngeal cancer surgeries. A further study is needed to establish efficacies of various rehabilitation programs based on the aspiration pattern described in this study.
In conclusion, the most common pattern observed in advanced T stage tongue cancer patients after glossectomy was aspiration after swallowing, not aspiration before swallowing due to impaired bolus control. Thus, postoperative aspiration pattern might not only result in inherent function degradation of the tongue being resected, but also lead to impairment of other swallowing mechanisms. In addition, resection of BOT at a degree greater than 25% for those with tongue and oropharyngeal cancer was a significant factor causing the combined type of aspiration. These findings may facilitate the counseling of patients before surgery and establishing a plan for post-operative swallowing rehabilitation.
ACKNOWLEDGMENTS
This work was supported by National Research Foundation of Korea (NRF) (grant numbers: 2021R1F1A1063088) and National University Research Fund (03-2021-0070). Since these funds were provided to the corresponding author for the purpose of basic research, there was no conflict of interest to declare.
Notes
Author Contribution
Conceptualization: all authors. Data curation: Bo Hae Kim, EunJae Chung. Formal analysis: all authors. Funding acquisition: EunJae Chung. Investigation: Bo Hae Kim, Eun-Jae Chung. Methodology: Eun-Jae Chung. Supervision: Eun-Jae Chung, Young-Soo Rho. Visualization: Bo Hae Kim. Writing—original draft: Bo Hae Kim. Writing—review & editing: Bo Hae Kim, Eun-Jae Chung.
REFERENCES
1. Goyal RK, Mashimo H. Physiology of oral, pharyngeal, and esophageal motility. [cited 2021 Feb 20]. Available from: URL: https://www.nature.com/gimo/contents/pt1/full/gimo1.html.
2. Pauloski BR. Rehabilitation of dysphagia following head and neck cancer. Phys Med Rehabil Clin N Am. 2008; 19(4):889–928.

3. Kalavrezos N, Cotrufo S, Govender R, Rogers P, Pirgousis P, Balasundram S, et al. Factors affecting swallow outcome following treatment for advanced oral and oropharyngeal malignancies. Head Neck. 2014; 36(1):47–54.

4. Kreeft AM, van der Molen L, Hilgers FJ, Balm AJ. Speech and swallowing after surgical treatment of advanced oral and oropharyngeal carcinoma: A systematic review of the literature. Eur Arch Otorhinolaryngol. 2009; 266(11):1687–98.

5. Govender R, Smith CH, Gardner B, Barratt H, Taylor SA. Improving swallowing outcomes in patients with head and neck cancer using a theory-based pretreatment swallowing intervention package: Protocol for a randomised feasibility study. BMJ Open. 2017; 27(7(3)):e014167.

6. Borggreven PA, Verdonck-de Leeuw I, Rinkel RN, Langendijk JA, Roos JC, David EF, et al. Swallowing after major surgery of the oral cavity or oropharynx: A prospective and longitudinal assessment of patients treated by microvascular soft tissue reconstruction. Head Neck. 2007; 29(7):638–47.

7. Logemann JA. Critical factors in the oral control needed for chewing and swallowing. J Texture Stud. 2014; 45(3):173–9.

8. Kronenberger MB, Meyers AD. Dysphagia following head and neck cancer surgery. Dysphagia. 1994; 9(4):236–44.

9. Huang ZS, Chen WL, Huang ZQ, Yang ZH. Dysphagia in tongue cancer patients before and after surgery. J Oral Maxillofac Surg. 2016; 74(10):2067–72.

10. McCulloch TM, Jaffe D. Head and neck disorders affecting swallowing. [cited 2021 Feb 20]. Available from: URL: https://www.nature.com/gimo/contents/pt1/full/gimo36.html.
11. Sakamoto Y, Yanamoto S, Ota Y, Furudoi S, Komori T, Umeda M. Magnitude of myocutaneous flaps and factors associated with loss of volume in oral cancer reconstructive surgery. J Oral Maxillofac Surg. 2016; 74(3):644–9.

12. Fattori B, Giusti P, Mancini V, Grosso M, Barillari MR, Bastiani L, et al. Comparison between videofluoroscopy, fiberoptic endoscopy and scintigraphy for diagnosis of oro-pharyngeal dysphagia. Acta Otorhinolaryngol Ital. 2016; 36(5):395–402.
13. Hiss SG, Postma GN. Fiberoptic endoscopic evaluation of swallowing. Laryngoscope. 2003; 113(8):1386–93.

14. Leder SB, Karas DE. Fiberoptic endoscopic evaluation of swallowing in the pediatric population. Laryngoscope. 2000; 110(7):1132–6.

15. Langmore SE. Endoscopic evaluation of oral and pharyngeal phases of swallowing. [cited 2021 Feb 21]. Available from: URL: https://www.nature.com/gimo/contents/pt1/full/gimo28.html.
16. Zuydam AC, Rogers SN, Brown JS, Vaughan ED, Magennis P. Swallowing rehabilitation after oro-pharyngeal resection for squamous cell carcinoma. Br J Oral Maxillofac Surg. 2000; 38(5):513–8.

17. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11(2):93–8.

18. Hirano M, Kuroiwa Y, Tanaka S, Matsuoka H, Sato K, Yoshida T. Dysphagia following various degrees of surgical resection for oral cancer. Ann Otol Rhinol Laryngol. 1992; 101(2 Pt 1):138–41.

19. Furia CL, Carrara-de Angelis E, Martins NM, Barros AP, Carneiro B, Kowalski LP. Video fluoroscopic evaluation after glossectomy. Arch Otolaryngol Head Neck Surg. 2000; 126(3):378–83.

20. Giannitto C, Preda L, Zurlo V, Funicelli L, Ansarin M, Di Pietro S, et al. Swallowing disorders after oral cavity and pharyngolaryngeal surgery and role of imaging. Gastroenterol Res Pract. 2017; 2017:7592034.

Fig. 1.
Aspiration pattern based on fiberoptic evaluation of swallowing. A: Premature spillage (asterisk) into the pharynx before swallowing caused by failure of bolus control after total glossectomy including the BOT reconstructed with an anterolateral thigh free flap. B: Aspiration after swallowing due to pyriform sinus residue after wide excision including the BOT with an anterolateral thigh free flap reconstruction in oropharyngeal cancer. BOT, base of tongue.
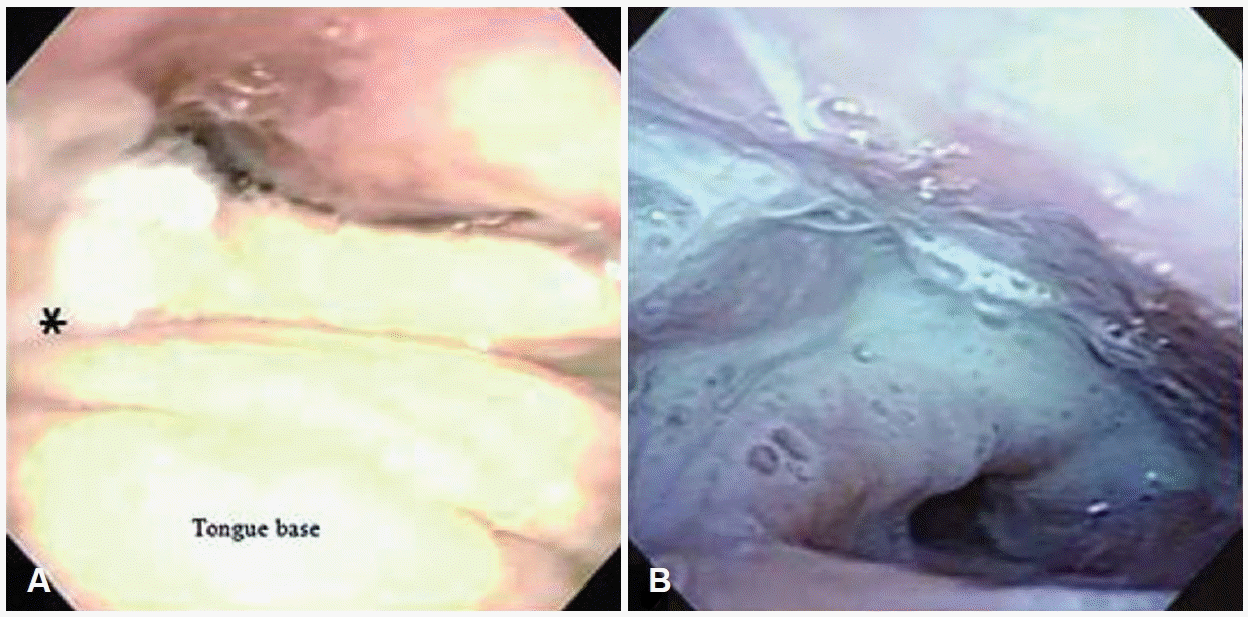
Fig. 2.
Aspiration patterns after tongue and oropharyngeal cancer surgery. A: The proportion of combined aspiration was significantly increased by simultaneous resection of BOT along with tongue cancer (p<0.001). B: The proportion of aspiration pattern in BOT preservation and BOT resection groups differed significantly (p=0.031). BOT, base of tongue.
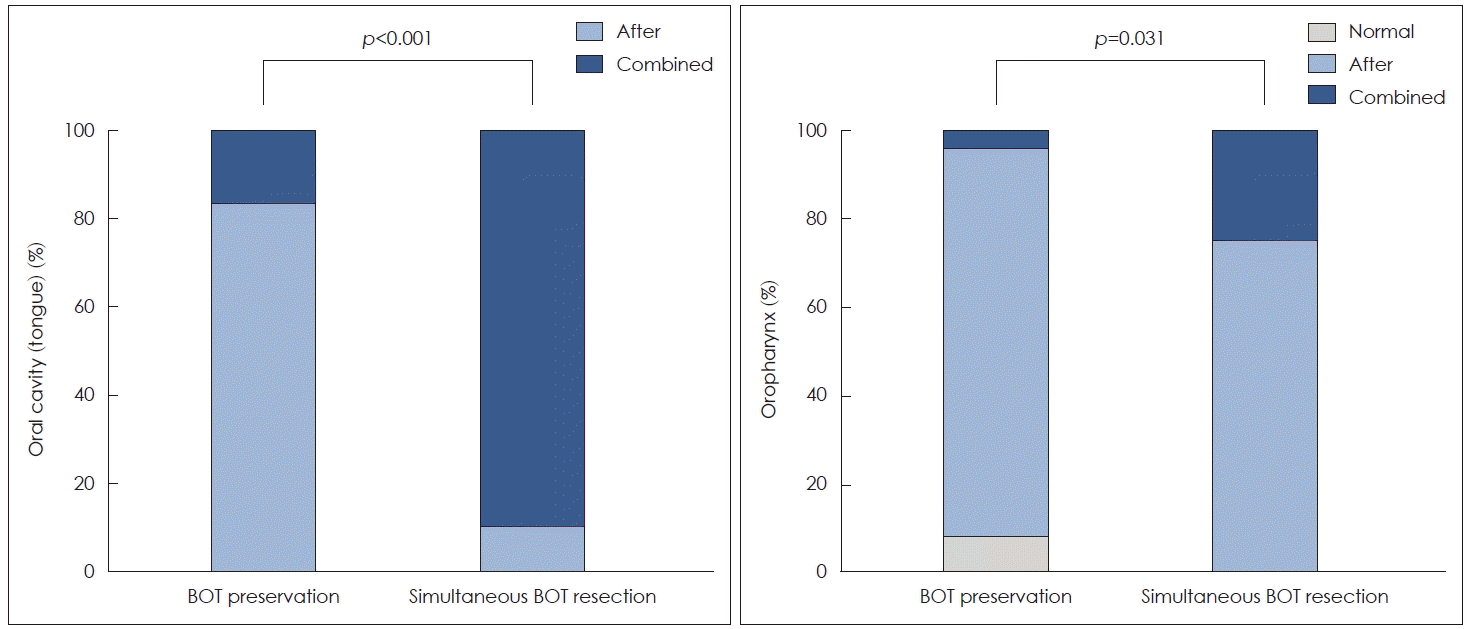
Table 1.
Demographic characteristics and types of surgery indicated for T3-T4 oral and oropharyngeal cancer
Table 2.
Aspiration pattern in patients with T3/T4 cancer of oral cavity and oropharynx determined via fiberoptic evaluation of swallowing




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download