Abstract
Notes
Ethics Statement
This study was approved by the Institutional Review Board with a waiver of informed consent (IRB No IEC-404/02.09.2016) and performed in accordance with the principles of the Declaration of Helsinki.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Author contributions
Conceptualization: PS. Data curation: PS. Formal analysis: PS. Supervision: DJ. Visualization: DJ. Writing—original draft: PS. Writing—review & editing: PS, AN, MKG, RR, SK, PSM, DJ. Approval of final manuscript: all authors.
References
Fig. 1.
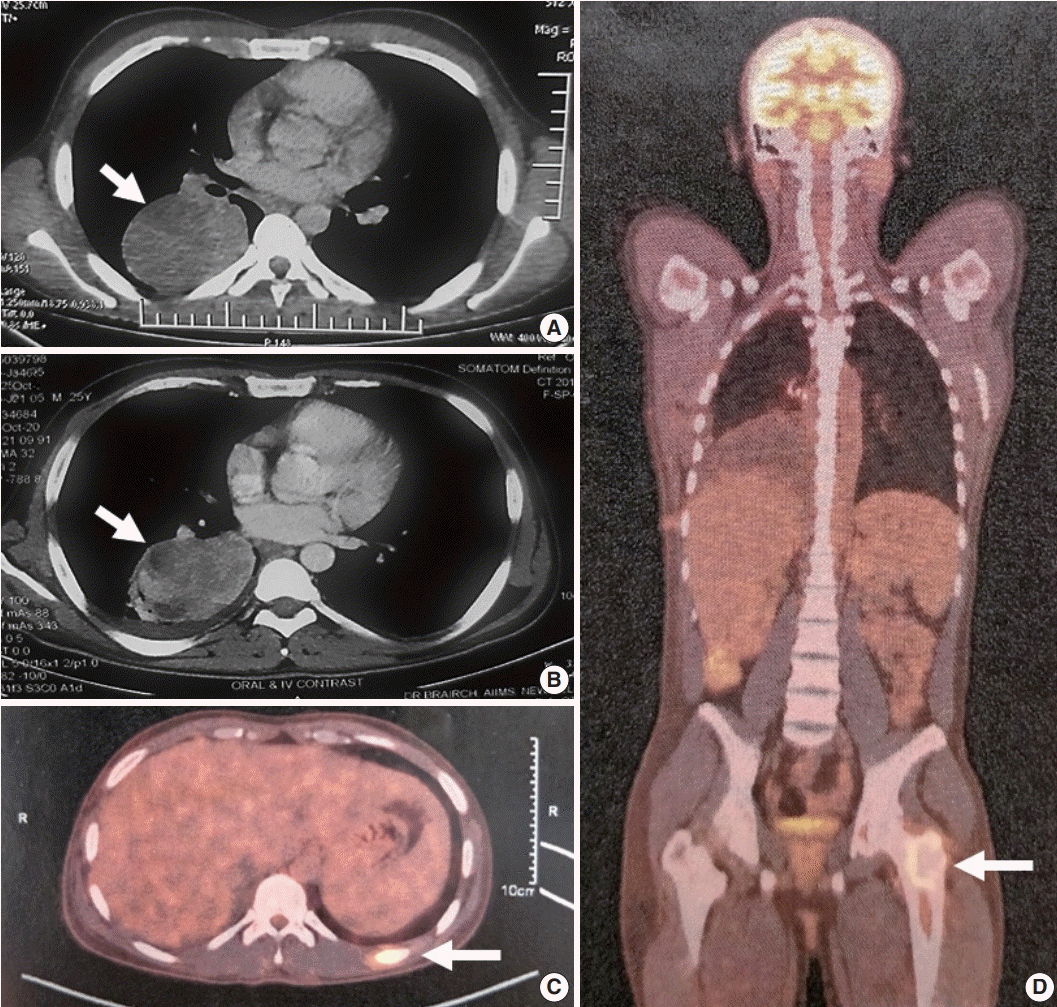
Fig. 2.
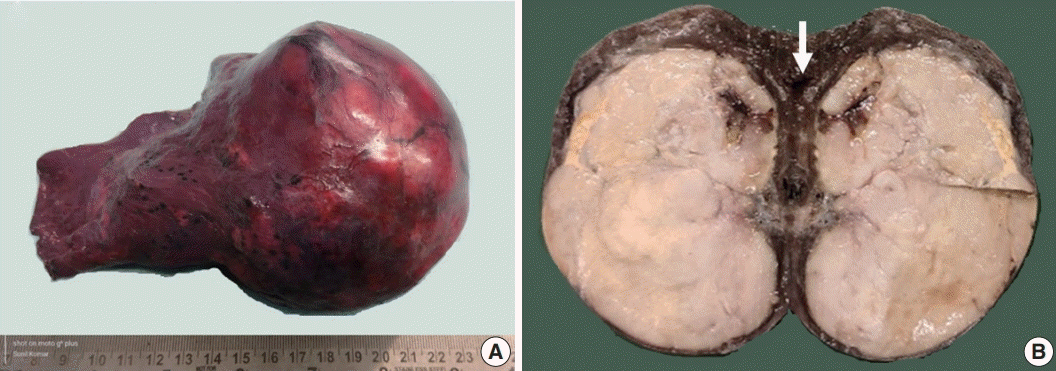
Fig. 3.
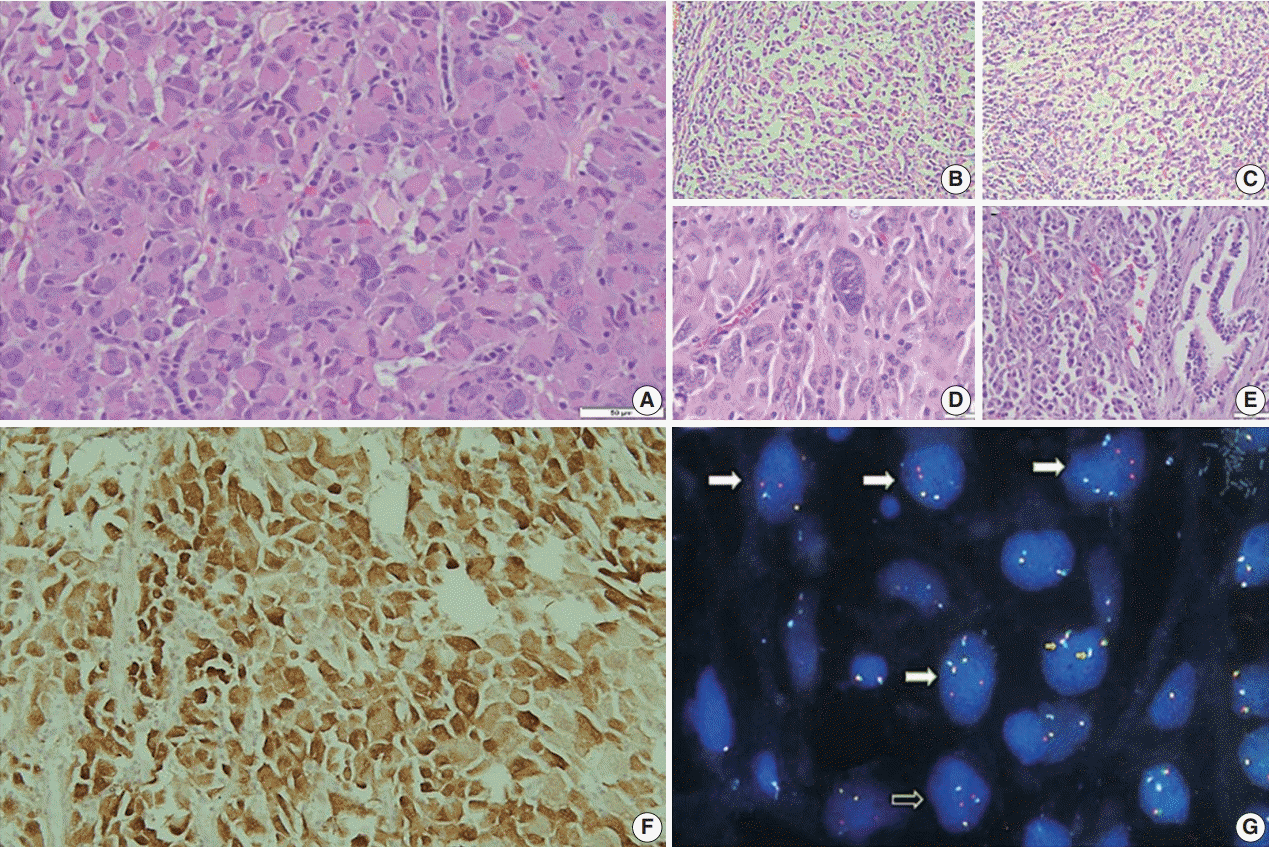
Table 1.
| No. | Study | Age (yr)/Sex | Anatomic site | Size (cm) | Clinical Presentation | Metastasis at presentation | Immunoprofile | Molecular confirmation (method) | Primary treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fu et al. [7] | 21/M | Left lower lobe lung | 10 | Weight loss, fatigue | None | Desmin+, ALK (c), CD30– | ALK rearrangement (FISH) | Lobectomy | Bone metastases (pelvis, vertebrae) and underwent laminectomy; death 4 mo after resection |
| 2 | Sarmiento et al. [8] | 71/M | Pleura based, left lower lobe | 12.5 | Dyspnoea, pleural effusion | None | NA | ALK rearrangement (FISH) | Lobectomy and adjuvant crizotinib | Progressed on crizotinib after 2 mo; near complete response to second line ALK inhibitor; remission after 1 yr at last follow-up |
| 3 | Kozu et al. [9] | 57/M | Pleural cavity/chest wall | NA | Pleural effusion and dyspnoea | N/A | Desmin+, cytokeratin+, ALK+ (c), CD30– | RANBP2-ALK fusion (PCR) | Crizotinib | NA |
| 4 | Present case | 25/M | Lung | 7 | Cough, loss of weight, fever, dyspnoea | Liver (solitary) | Desmin+, focal cytokeratin+, ALK+ (c), CD30– | ALK rearrangement (FISH) | Crizotinib | Complete resolution of liver metastases and stable pulmonary disease for 6 mo; bony metastases developed at 10 mo; lobectomy done and stable metastatic disease on crizotinib for 3 mo |
| Right lower lobe |
Table 2.
| Entity | Incidence as lung mass | Epithelial markers | Mesenchymal markers | Other markers | ALK protein expression | Commonest ALK fusions |
|---|---|---|---|---|---|---|
| IMT [2] | Common | Pan CK+/– | SMA+, Desmin+ | CD30– | Cytoplasmic (50%–60%) | TPM3-ALK, TPM4-ALK |
| ALCL [21] | Uncommon | EMA+ | SMA+, Desmin– | CD30+ | Nuclear and cytoplasmic | NPM-ALK |
| ALK+LBL [21] | Rare | EMA+ | – | CD138+, CD38+, Mum1/IRF1+, IgA+, Bob1+ | Cytoplasmic, nuclear and nucleolar | CLTC-ALK |
| ALK-SEC31A | ||||||
| ALK-rearranged NSCLC | Common | CK+ | – | Cytoplasmic | EML4-ALK [14] | |
| Melanoma | Extremely rare | CK– | – | S-100+, Melan A+, HMB45+, SOX10+ | ALKATI (seen in cutaneous melanoma) [22] | EML4-ALK [23] |
| Epithelioid mesothelioma [2] | Uncommon | CK5/6+/–, EMA+/– | Desmin– | Calretinin+/–, WT+, D2-40+ | – | – |
| Rhabdomyosarcoma | Rare | CK/EMA– | Desmin+, Actin+ | MyoD1+, Myogenin+ CD30– | Cytoplasmic | NPM-ALK [24], EML4-ALK [25] |
| Epithelioid leiomyosarcoma [1] | Rare | EMA+ | SMA+, Desmin+, h-caldesmon+ | CD34+/–CK+/–, EMA+/– | – | – |
| EIMS [2] | Rare | CK or EMA–/+ | Desmin+, SMA+ | CD30+ | Nuclear membrane or cytoplasmic with perinuclear accentuation | ALK-RRBP1 [3-5], ALK-RANBP2 |
| Pleomorphic carcinoma [2] | < 1% | NSCC component CK+, TTF-1 +, EMA+ | Vimentin+ | Surfactant protein A+, p53+ | Rare | – |
ALK, anaplastic lymphoma kinase; ALK ATI, anaplastic lymphoma kinase with alternative transcription initiation; IMT, inflammatory myofibroblastic tumor; CK/PanCK, cytokeratin; SMA, smooth muscle actin; TPM, tropomyosin; ALCL, anaplastic large cell lymphoma; EMA, epithelial membrane antigen; NPM, nucleophosmin; Mum1/IRF1, multiple myeloma 1/interferon regulatory factor 4 protein; CLTC, clathrin heavy chain; NSCLC, non–small cell lung carcinoma; EML4, echinoderm microtubule-associated protein-like 4; HMB45, human melanoma black 45; EIMS, epithelioid Inflammatory myofibroblastic sarcoma; RRBP1, ribosome binding protein 1; RANBP, Ran-binding protein; NSCC, non-small cell lung cancer; TTF-1, thyroid transcription factor 1.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download