Abstract
Notes
Ethics Statement
This case report was approved by the IRB of Chonnam Natinal University Hwasun Hospital (CNUHH-2021-240). Informed consent was obtained from the participant included in the study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: CC. Data curation: JYK, KHL, JHC. Investigation: JYK. Methodology: YJK, KHL, HSC. Supervision: CC. Visualization: JYK. Writing—original draft: JYK. Writing—review & editing: CC, JHC. Approval of final manuscript: all authors.
References
Fig. 1
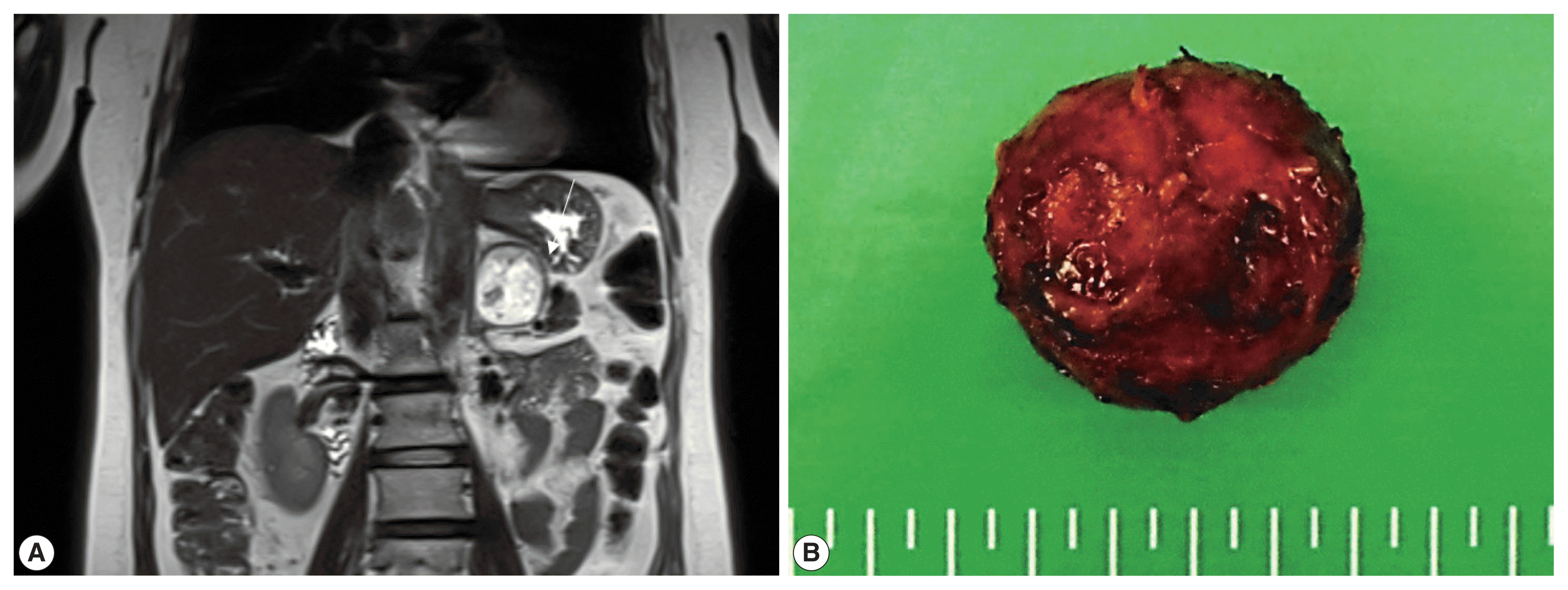
Fig. 2

Fig. 3

Table 1
| Case | Age (yr)/Sex | Clinical feature | Size/Weight | Microscopic findings | Immunohistochemical or special stain/EM | Association with VHL syndrome | Follow-up |
|---|---|---|---|---|---|---|---|
| Burns et al. (1987) [6] | 23/F | Erythrocytosis (hematocrit, 58%) | 5.0 cm | Abundant thin-walled blood vessels; stromal cells, large cells with vacuolated clear cytoplasm | Stromal cell with fat vacuole | Yes | NA |
| Itoh et. al. (1988) [7] | 49/M | Incidentally found; elevated noradrenalin (187.4 μg/day) and dopamine (2,308 μg/day) in urine | 2.7 cm | Abundant capillary structures; stromal cells, large ovoid | NSE (+), fat stain (+) | No | NA |
| Nonaka et al. (2007) [3] | 30/F | Cerebellar and spinal HB, bilateral adrenal HB | 0.9 cm; 0.5 cm | Focal cavernous vascular spaces; stromal cells, vacuolated large with amphophilic cytoplasm, round to oval nuclei | S-100 protein (+, focal), chromogranin (+), CD31 (−), CD34 (−) | Yes | NA |
| Browning and Parker (2008) [8] | 30/M | Bilateral adrenal HB, spinal and cerebellar HB, pancreatic and renal cysts, cystic renal cell carcinoma (suspicious) | Not identified (12.5 g; 8.3 g) | Abundant capillaries; stromal spindled cells. Stromal cells; large, intimately connected with vascular walls | Chromogranin (+), vimentin (+), S-100 protein (−) | Yes | NA |
| Current case | 54/F | Incidentally found | 4.2 cm | Abundant capillaries; stromal cells, vacuolated or amphophilic cytoplasm | CD34 (−), CD31 (−), inhibin-alpha (−), EMA (−), CK (−), CA-9 (−), CD10 (−), S-100 protein (+), synaptophysin (+), NSE (+) | No | Alive (1 yr) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download