1. Yang DJ, Park SK, Kim JH, Heo JW, Lee YS, Uhm YH. Effect of changes in postural alignment on foot pressure and walking ability of stroke patients. J Phys Ther Sci. 2015; 27:2943–5.

2. Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006; 12:290–5.

3. Walsh ME, Horgan NF, Walsh CD, Galvin R. Systematic review of risk prediction models for falls after stroke. J Epidemiol Community Health. 2016; 70:513–9.

4. Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997; 77:812–9.

5. Cho KH, Bok SK, Kim YJ, Hwang SL. Effect of lower limb strength on falls and balance of the elderly. Ann Rehabil Med. 2012; 36:386–93.

6. Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009; 64:896–901.

7. Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012; 41:299–308.

8. Anstey KJ, von Sanden C, Luszcz MA. An 8-year prospective study of the relationship between cognitive performance and falling in very old adults. J Am Geriatr Soc. 2006; 54:1169–76.

9. Czernuszenko A, Czlonkowska A. Risk factors for falls in stroke patients during inpatient rehabilitation. Clin Rehabil. 2009; 23:176–88.

10. Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: factors associated with high risk. Arch Phys Med Rehabil. 2002; 83:329–33.

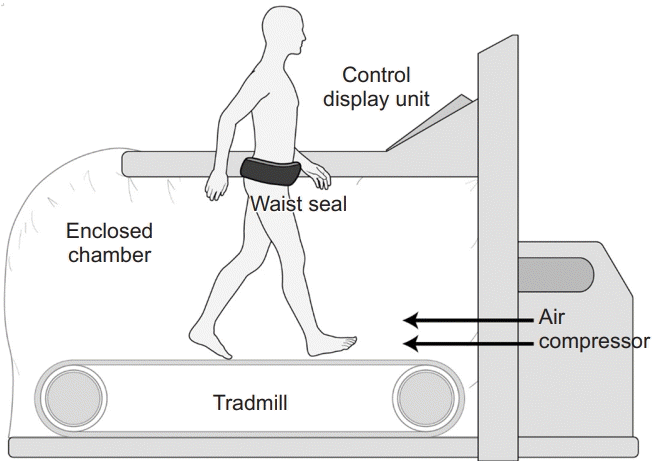
11. Lathan C, Myler A, Bagwell J, Powers CM, Fisher BE. Pressure-controlled treadmill training in chronic stroke: a case study with AlterG. J Neurol Phys Ther. 2015; 39:127–33.
12. Baizabal-Carvallo JF, Alonso-Juarez M, Fekete R. Antigravity treadmill training for freezing of gait in Parkinson’s disease. Brain Sci. 2020; 10:739.

13. Peeler J, Leiter J, MacDonald P. Effect of body weight-supported exercise on symptoms of knee osteoarthritis: a follow-up investigation. Clin J Sport Med. 2020; 30:e178. –e185.
14. Berthelsen MP, Husu E, Christensen SB, Prahm KP, Vissing J, Jensen BR. Anti-gravity training improves walking capacity and postural balance in patients with muscular dystrophy. Neuromuscul Disord. 2014; 24:492–8.

15. Hargens AR, Whalen RT, Watenpaugh DE, Schwandt DF, Krock LP. Lower body negative pressure to provide load bearing in space. Aviat Space Environ Med. 1991; 62:934–7.
16. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986; 80:429–34.

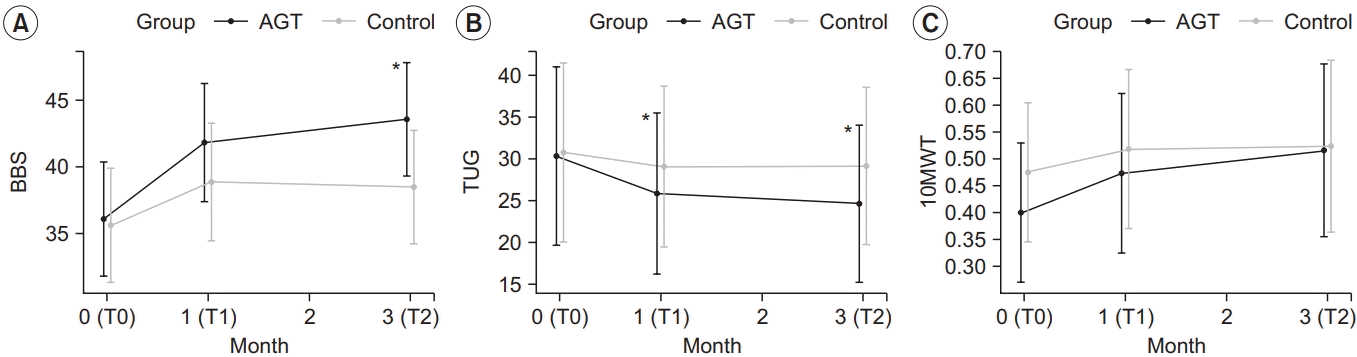
17. Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004; 52:1343–8.

18. Canbek J, Fulk G, Nof L, Echternach J. Test-retest reliability and construct validity of the Tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther. 2013; 37:14–9.

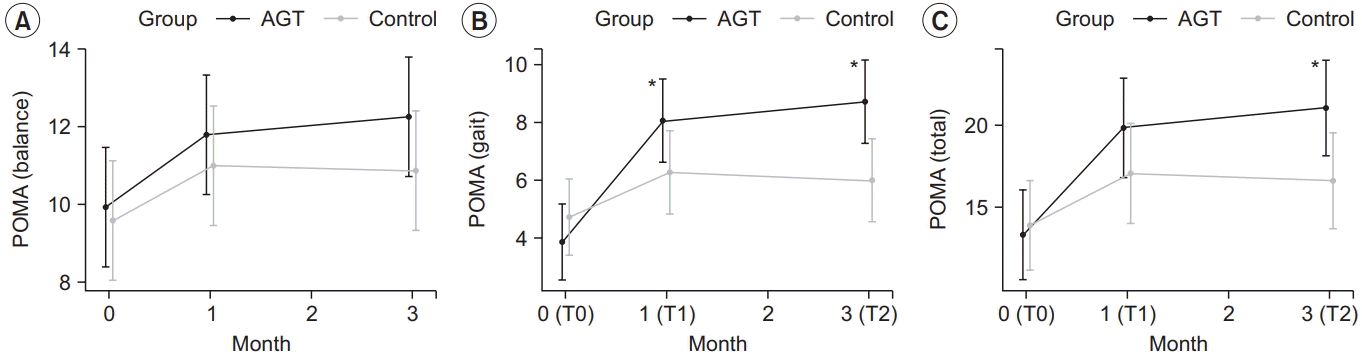
19. Maeda N, Kato J, Shimada T. Predicting the probability for fall incidence in stroke patients using the Berg Balance Scale. J Int Med Res. 2009; 37:697–704.

20. Geiger RA, Allen JB, O’Keefe J, Hicks RR. Balance and mobility following stroke: effects of physical therapy interventions with and without biofeedback/forceplate training. Phys Ther. 2001; 81:995–1005.

21. Srivastava A, Taly AB, Gupta A, Kumar S, Murali T. Post-stroke balance training: role of force platform with visual feedback technique. J Neurol Sci. 2009; 287:89–93.

22. Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther. 2012; 36:166–72.

23. Parvin S, Taghiloo A, Irani A, Mirbagheri MM. Therapeutic effects of anti-gravity treadmill (AlterG) training on reflex hyper-excitability, corticospinal tract activities, and muscle stiffness in children with cerebral palsy. IEEE Int Conf Rehabil Robot. 2017; 2017:485–90.

24. Dadashi F, Kharazi MR, Lotfian M, Shahroki A, Mirbagheri A, Mirbagheri MM. The effects of lower body positive pressure treadmill training on dynamic balance of children with cerebral palsy. Annu Int Conf IEEE Eng Med Biol Soc. 2018; 2018:2487–90.

25. Aras B, Yasar E, Kesikburun S, Turker D, Tok F, Yilmaz B. Comparison of the effectiveness of partial body weight-supported treadmill exercises, robotic-assisted treadmill exercises, and anti-gravity treadmill exercises in spastic cerebral palsy. Turk J Phys Med Rehabil. 2019; 65:361–70.

26. Hesse S. Treadmill training with partial body weight support after stroke: a review. NeuroRehabilitation. 2008; 23:55–65.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download