1. Bulfone G, Del Negro F, Del Medico E, Cadorin L, Bressan V, Stevanin S. Rehabilitation strategies for low anterior resection syndrome: a systematic review. Ann Ist Super Sanita. 2020; 56:38–47.
2. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012; 13:e403. –8.

3. Dulskas A, Smolskas E, Kildusiene I, Samalavicius NE. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis. 2018; 33:251–60.

4. Emmertsen KJ, Laurberg S. Bowel dysfunction after treatment for rectal cancer. Acta Oncol. 2008; 47:994–1003.

5. Camilleri-Brennan J, Steele RJ. Prospective analysis of quality of life and survival following mesorectal excision for rectal cancer. Br J Surg. 2001; 88:1617–22.

6. van Duijvendijk P, Slors JF, Taat CW, van Tets WF, van Tienhoven G, Obertop H, et al. Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol. 2002; 97:2282–9.

7. Pieniowski EH, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ, et al. Low anterior resection syndrome and quality of life after sphincter-sparing rectal cancer surgery: a long-term longitudinal follow-up. Dis Colon Rectum. 2019; 62:14–20.

8. Madoff RD, Parker SC, Varma MG, Lowry AC. Faecal incontinence in adults. Lancet. 2004; 364:621–32.

9. Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003; 125:1320–9.

10. Maeda Y, Matzel K, Lundby L, Buntzen S, Laurberg S. Postoperative issues of sacral nerve stimulation for fecal incontinence and constipation: a systematic literature review and treatment guideline. Dis Colon Rectum. 2011; 54:1443–60.

11. Michelsen HB, Christensen P, Krogh K, Rosenkilde M, Buntzen S, Theil J, et al. Sacral nerve stimulation for faecal incontinence alters colorectal transport. Br J Surg. 2008; 95:779–84.

12. Giani I, Novelli E, Martina S, Clerico G, Luc AR, Trompetto M, et al. The effect of sacral nerve modulation on cerebral evoked potential latency in fecal incontinence and constipation. Ann Surg. 2011; 254:90–6.

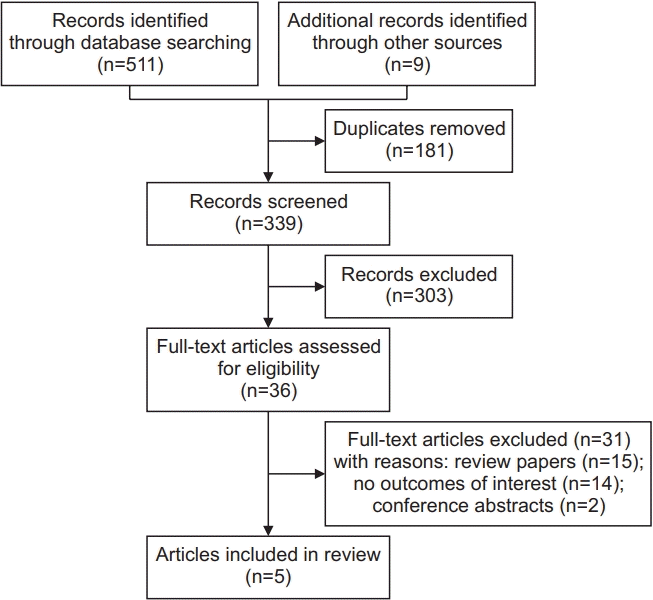
13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

16. Vigorita V, Rausei S, Troncoso Pereira P, Trostchansky I, Ruano Poblador A, Moncada Iribarren E, et al. A pilot study assessing the efficacy of posterior tibial nerve stimulation in the treatment of low anterior resection syndrome. Tech Coloproctol. 2017; 21:287–93.

17. Enriquez-Navascues JM, Labaka-Arteaga I, Aguirre-Allende I, Artola-Etxeberria M, Saralegui-Ansorena Y, Elorza-Echaniz G, et al. A randomized trial comparing transanal irrigation and percutaneous tibial nerve stimulation in the management of low anterior resection syndrome. Colorectal Dis. 2020; 22:303–9.

18. Marinello FG, Jimenez LM, Talavera E, Fraccalvieri D, Alberti P, Ostiz F, et al. Percutaneous tibial nerve stimulation in patients with severe low anterior resection syndrome: randomized clinical trial. Br J Surg. 2021; 108:380–7.

19. Cuicchi D, Di Fabio F, Guido A, Llimpe FL, Morganti AG, Ardizzoni A, et al. Randomized pilot trial of percutaneous posterior tibial nerve stimulation versus medical therapy for the treatment of low anterior resection syndrome: one-year follow-up. Dis Colon Rectum. 2020; 63:1602–9.

20. Altomare DF, Picciariello A, Ferrara C, Digennaro R, Ribas Y, De Fazio M. Short-term outcome of percutaneous tibial nerve stimulation for low anterior resection syndrome: results of a pilot study. Colorectal Dis. 2017; 19:851–6.

21. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012; 255:922–8.
22. Altomare DF, Spazzafumo L, Rinaldi M, Dodi G, Ghiselli R, Piloni V. Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Colorectal Dis. 2008; 10:84–8.

23. Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999; 44:77–80.

24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993; 36:77–97.

25. Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999; 42:1525–32.
26. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–76.

27. Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int J Surg. 2018; 56:234–41.

28. Visser WS, Te Riele WW, Boerma D, van Ramshorst B, van Westreenen HL. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol. 2014; 30:109–14.

29. Ramage L, Qiu S, Kontovounisios C, Tekkis P, Rasheed S, Tan E. A systematic review of sacral nerve stimulation for low anterior resection syndrome. Colorectal Dis. 2015; 17:762–71.

30. de Miguel M, Oteiza F, Ciga MA, Armendariz P, Marzo J, Ortiz H. Sacral nerve stimulation for the treatment of faecal incontinence following low anterior resection for rectal cancer. Colorectal Dis. 2011; 13:72–7.

31. Leroi AM, Siproudhis L, Etienney I, Damon H, Zerbib F, Amarenco G, et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol. 2012; 107:1888–96.

32. Knowles CH, Horrocks EJ, Bremner SA, Stevens N, Norton C, O’Connell PR, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group, randomised controlled trial. Lancet. 2015; 386:1640–8.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download