Abstract
Objective
To investigate the association of trunk fat and muscle composition, lumbar disc space narrowing, and low back pain in middle-aged farmers.
Methods
Fat and muscle areas were identified using standard Hounsfield unit ranges for adipose tissue and skeletal muscle with computed tomography images at the mid-L4 vertebral level. Trunk fat mass, muscle mass, and fat/muscle mass ratio were calculated. Low back pain was assessed using the Oswestry Disability Index (ODI). The L4/5-disc space and low back pain were also assessed.
Results
Male had a higher total trunk, back, psoas, and abdominal muscle mass, and visceral fat; female had a higher subcutaneous fat mass and fat/muscle ratio. Pearson correlation coefficients with ODI for waist circumference, total fat mass, visceral fat mass, and fat/muscle ratio were all significant in female; only the fat/muscle ratio was significant in male. Pearson correlation coefficients with L4/5-disc space narrowing grades for visceral fat mass, total, back, and psoas muscle mass, and fat/muscle ratio, were all significant in female; total and back muscle mass, and fat/muscle ratio in male.
The low back is the most common site of musculoskeletal pain for farmers [1], and low back pain (LBP) appears to be more prevalent in farmers than in other workers with fewer physical occupational demands [2]. The low back is affected significantly during agricultural work. In particular, the lumbar spine is affected by lifting of heavy weights, harvesting, and planting [3]. Back muscles play an important role in maintaining the stability of the lumbar spine, while the trunk muscle mass has been reported to be associated with back pain. Abdominal trunk muscle weakness is associated with chronic LBP [4]. Pain could be associated with the trunk muscle mass. Preventing back pain is important for farmers, and research on the trunk muscle mass of farmers is required.
While previous studies have investigated the association between a decrease in trunk muscle mass and LBP, the results are conflicting [5]. Decreased muscle mass has been reported to be associated with LBP [6], while muscle density, intramuscular fat mass, or paraspinal fat infiltration, but not the area of muscle in the lower back, were also related to LBP [7,8]. There have been no studies on the relationship between back muscle mass and back pain in farmers, although there are several studies on the relationship between trunk muscles and LBP in the general population.
The association between trunk fat mass and LBP has been reported, but the mechanism is unclear [9]. Not only overweight assessed by body mass index (BMI) [10], but excess fat mass around the abdomen [9] and fat-tolean-mass ratio [9] were also associated with LBP. There has been little research on the relationship between the distribution of subcutaneous and visceral fat in the abdomen and the occurrence of LBP.
Computed tomography (CT) could provide cross-sectional area of fat-free paraspinal muscle areas, and separate measurements for both subcutaneous and visceral fat [11]. Radiological attenuation assessed in Hounsfield units (HU) is used to differentiate the muscular tissue from adipose tissue [12]. The L3 to L4 level is frequently chosen as the target level for muscle mass measurements as muscle mass is greatest here [13]. The L4 level is also frequently assessed to determine the association between trunk muscle mass and LBP [11,14,15]. Visceral fat measured in a single slice CT scan at the L4 level has been shown to correlate significantly with total abdominal visceral fat volume [16].
We aimed to investigate the association between the CT-based trunk muscle/fat mass at the mid-L4 level and LBP and narrowing of the lumbar disc space in middleaged adults.
In total, 446 eligible participants were selected from the Farmers’ Cohort for Agricultural Work-Related Musculoskeletal Disorders study conducted from 2013 to 2014, and 339 new participants were selected from individuals farming in Gangwon Province, South Korea. After exclusion of non-active farmers, 590 were selected to participate in this study. A further 21 participants due to a history of spine surgery and 10 participants with compression fracture were excluded. Finally, 463 middle-aged (40–64 years old) participants were included in the final analysis. Since factors that can be affected by age (e.g., body composition and disc degeneration) were included in the analysis, the relationships between body fat and muscle ratio, intervertebral disc degeneration, and LBP, were evaluated in middle-aged people. Farming was classified into four types: rice farming (e.g., rice), dry fields farming (e.g., corn, potato), greenhouses farming (e.g., cucumber, tomato), and orchards farming (e.g., apple, peach). This study was approved by the Institutional Review Board of Kangwon National University Hospital (No. 2016-03-008) and written informed consent was obtained from all the participants. This study was also registered with the Clinical Research Information Service.
CT images at the mid-L4 vertebral level were obtained for each participant using a Philips MX 8000 IDT CT scanner (Philips Medical Systems, Cleveland, OH, USA), with tube voltage set at 120 kV, exposure at 200 mAs, and slice thickness at 1 mm. Images were taken from ten consecutive slices of 1-mm thickness, covering a total thickness of 10 mm. During the CT examination, patients kept both hips in a neutral position to avoid any effect of hip flexion on the cross-sectional area (CSA) of the muscle. Pre-defined radiation attenuation ranges were used to demarcate adipose tissue (from -190 HU to -30 HU) and muscle (-29 HU to +150 HU) [12], and total muscle mass (TMM, cm3) and total fat mass (TFM, cm3) were automatically derived using image processing software (Extended Brilliance Workspace version 4.5.3; Philips Healthcare, Best, The Netherlands) (Fig. 1). The TMM was subdivided by manually outlining the range of interest, psoas muscle mass (PMM, cm3) and back muscle mass (BMM, cm3). Abdominal muscle mass (AMM, cm3) was calculated by subtracting the PMM and BMM from the TMM. BMM consists of the multifidus, iliocostalis lumborum, longissimus, and quadratus lumborum muscles. From the TFM, visceral fat mass (VFM, cm3) was computed by manually outlining the inner abdominal wall. Subcutaneous fat mass (SFM, cm3) was calculated as TFM minus VFM. One technician performed all the scans and image processing (Fig. 1). Trunk fat/muscle ratio was calculated by dividing TFM by TMM.
LBP was assessed using the Oswestry Disability Index (ODI). The ODI is a self-administered 10-item questionnaire covering intensity of pain and disability experienced in standing, personal care, sleeping, lifting, sex life, walking, social life, sitting, or traveling. The index was translated into Korean, and its validity and reliability have been assessed [17]. Scores for each item range from 0 (no pain or disability) to 5 (severe pain or disability). Scores are reported as percentages according to the following formula: total score (0–50)/maximum score (50)×100 [18].
The Korean version of the International Physical Activity Questionnaire (IPAQ) was used to evaluate participant’s physical activity levels [19]. The seven items of the IPAQ identifies the total time (in minute) spent on physical activity, including inactivity, walking, moderateintensity, and vigorous-intensity activities, over the last 7 days. Replies were converted to metabolic equivalent task minutes per week (MET-min/wk) according to the IPAQ scoring method. An average METs was derived for each type of activity. The following MET values were used: walking=3.3 METs, moderate physical activity=4.0 METs, and vigorous physical activity=8.0 METs. The total physical activity was calculated as the sum of the METmin/ wk values derived from walking, moderate activity, and vigorous activity.
Assessment of the L3/4, L4/5, and L5/S1-disc space was completed through a lateral spine radiograph by a radiologist who was blinded to the participant’s information. Intervertebral disc height was measured as a percentage of the adjacent discs and graded according to a semi-quantitative score—grade 0, normal; grade 1, mild (>75%); grade 2, moderate (>50%); grade 3, severe (>25%); grade 4, very severe (<25%)—as described by Mimura et al. [20]. Spondylolisthesis was also evaluated by X-ray findings.
Demographic data, anthropometric measurements, LBP, disc space narrowing, and trunk body composition results were compared between male and female using t-test or χ2 test. ODI scores were not significantly different between farming types when analyzed using one-way ANOVA. Pearson correlation coefficients (r) were used to analyze the association between anthropometric measurements and trunk fat/muscle mass with ODI scores. The association of anthropometric measurements and trunk fat/muscle mass with L3/4-, L4/5-, and L5/S1-disc space narrowing and presence of spondylolisthesis was also analyzed using Spearman correlation coefficients (rho).
The participants’ characteristics are presented in Table 1. The distribution of farming types according to the major crop were: rice, 31 (6.7%); dry fields, 174 (37.6%); greenhouses, 210 (45.4%); and orchards, 48 (10.4%).
Female showed higher ODI scores (p<0.001), shorter height (p<0.001), less body weight (p<0.001), and thinner waist circumference (p<0.001) than those in male. CT results showed higher VFM in male (p<0.001) and higher SFM in female (p<0.001). Male had higher TMM, BMM, PMM, and AMM (p<0.001). Fat/muscle ratio was significantly higher in female (2.63) than in male (1.72) (p<0.001). The distribution of farming types was significantly different between sexes (p<0.001): more female participated in dry fields farming, whereas more male participated in rice and orchards farming. Grades of L5/ S1-disc space narrowing also showed different distribution between the sexes (p=0.002). Grades of L4/5-disc space narrowing showed different distribution between the sexes, but the difference was not statistically significant (p=0.050). Finally, grades of L3/4-disc space narrowing, age and BMI were not significantly different between the sexes (Table 1).
In the past year, 149 (32.2%) study participants responded that they had seen a doctor at the hospital due to back pain, and among them, 49 had to temporarily stop work due to pain.
ODI scores were not significantly different between farming types (p=0.284): rice, 10.7±12.4; dry fields, 13.1±13.4; greenhouses, 10.5±12; orchards, 11.8±12.3.
Pearson correlation coefficients with the ODI score were significant for waist circumference (r=0.178, p=0.004), TFM (r=0.124, p=0.048), VFM (r=0.151, p=0.015), and fat/muscle ratio (r=0.123, p=0.049) in female. In male farmers, fat/muscle ratio and ODI score showed a positive correlation, but did not reach statistical significance (r=0.122, p=0.08). The ODI score was not related to any of the muscle mass values or BMI (Table 2).
Table 3 shows the relationship between anthropometric and trunk fat/muscle parameters with physical activity. Pearson correlation coefficients with the IPAQ score were significant for TMM (r=0.152, p=0.015) and BMM (r=0.167, p=0.007) in female. In male, physical activity was not significantly related with fat or muscle mass.
Spearman correlation coefficients with narrowing of the L3/4-disc space were VFM (rho=0.125, p=0.046), TMM (rho=-0.138, p=0.026), and BMM (rho=-0.227, p<0.001) in female; BMM (rho=-0.146, p=0.037) in male (Table 4).
Spearman correlation coefficients with narrowing of the L4/5-disc space were waist circumference (rho=0.136, p=0.029), TFM (rho=0.134, p=0.032), VFM (rho=0.127, p=0.042), TMM (rho=-0.252, p<0.001), BMM (rho=-0.314, p<0.001), PMM (rho=-0.199, p=0.001), and fat/muscle ratio (rho=0.267, p<0.001) in female; TMM (rho=-0.168 , p=0.016), BMM (rho=-0.271, p<0.001), PMM (rho=-0.166, p=0.017), and fat/muscle ratio (rho=0.166, p=0.017) in male (Table 4).
Spearman correlation coefficients with narrowing of the L5/S1-disc space were height (rho=0.16, p=0.01), weight (rho=0.129, p=0.039), VFM (rho=0.167, p=0.007), and PMM (rho=-0.133, p=0.034) in female; height (rho=0.15, p=0.032) and BMM (rho=-0.144, p=0.039) in male (Table 4).
Spearman correlation coefficients with spondylolisthesis were BMI (rho=0.144, p=0.039), waist circumference (rho=0.143, p=0.04), and SFM (rho=0.163, p=0.02) in male. In female, the presence of spondylolisthesis was not significantly related with fat or muscle mass. In addition, the presence of spondylolisthesis was not related to any of the muscle mass values (Table 4).
The results of this study showed a significantly different pattern of trunk muscle/fat mass between male and female. Total trunk, back, psoas, and abdominal muscle mass were all higher in male. Total trunk fat mass was not significantly different between the sexes, while trunk fat distribution was: visceral fat was significantly higher in male and subcutaneous fat was significantly higher in female. Consequently, the trunk fat/muscle ratio was significantly higher in female than that in male. LBP was significantly associated with fat mass parameters and fat/ muscle ratio in female, while none of the muscle mass results, nor BMI, were significantly related with LBP. Disc space narrowing of L3/4, L4/5, and L5/S1 levels, were associated with increased fat mass, reduced muscle mass, and increased fat/muscle ratio.
Obesity, especially abdominal fat, seems to be associated with developing LBP. In our study, BMI was not significantly related with LBP, while abdominal fat mass parameters (waist circumference, total fat mass, visceral fat mass, and trunk fat/muscle ratio) were significantly related with LBP in female farmers. In previous studies, BMI was analyzed as cut-off points to determine the degree of overweight [10]. Contrary to the results of previous studies, in our study, the correlation was analyzed as a continuous variable without using cut-off. Among the indicators of obesity, waist circumference in particular was highly correlated with LBP [21]. This is consistent with the results of our study.
Both BMI and waist circumference are indirect measurements of fat mass, because body weight includes both muscle and fat mass, and the BMI increases when the muscle mass increases. The waist circumference includes abdominal fat, internal organs, and trunk muscles. Compared to previous studies that indirectly measured the amount of fat and muscle in the abdomen by body weight, waist circumference, we directly measured the amount of muscle and fat in the trunk using abdominal CT. We measured abdominal fat (both visceral and subcutaneous) using CT images of the L4 lumbar level. The fat mass of the L4 level measured by this method is proportional to the fat mass of the entire abdomen [16]. Our study showed that the amount of visceral fat in the abdomen was significantly related to LBP. Similarly, abdominal fat-related variables, including waist-to-hip ratio, and percentage of trunk fat mass/weight, were significantly linked to LBP [22]. Among obese people, it has been reported that the intensity of back pain increases as the amount of fat in the android region increases compared to the amount of fat in the gynoid region [9]. Although the mechanism between obesity and LBP has not been identified, it can be assumed that the fat distribution in the abdomen may be an aggravating factor in LBP. An increase in BMI was shown to be significantly related to an increase in lumbar lordosis [23], possibly due to abdominal fat placing a mechanical load on the spine. Obese individuals with chronic LBP showed a higher degree of spinal impairment compared to that in individuals without [24].
The results of this study showed that disc space narrowing and spondylolisthesis of the lumbar spine were significantly related to muscle mass as well as fat mass. Reduced trunk muscle mass (total and back) and increased fat/muscle ratio showed significant association with narrowing of the L4/5-disc space in both male and female. Increased fat mass (visceral) was associated with narrowing of the L4/5-disc space in female. In adjacent lumbar disc levels, similar associations with L4 level fat and muscle mass were observed: increased fat mass (visceral) and decreased muscle mass (total and back) in L3/4-disc space narrowing; and increased fat mass (visceral) and decreased muscle mass (back and psoas) in L5/S1-disc space narrowing. Also, lumbar spondylolisthesis was associated with fat parameters in male: BMI, waist circumference, subcutaneous fat mass, and trunk fat/muscle ratio.
Damage or disruption to the nerves of the back muscle leads to muscular atrophy. An experimental study showed that the paraspinal muscles atrophied rapidly after spinal nerve root injury in pigs compared to sham injury [25]. Similarly, a case-control study reported that patients with lumbar radiculopathy showed significantly reduced back muscle area compared to control patients [26]. The same study showed that lumbar disc herniation alone also reduced the back muscle area, to a similar extent as radiculopathy [26]. Symptomatic spinal stenosis has been reported to result in greater paraspinal muscle atrophy compared to asymptomatic controls, while LBP alone did not [27]. Narrowing of the lumbar disc space [28], degenerative lumbar flat back [29], and degenerative kyphosis [30] have also all been associated with a lower CSA of the back muscles. The results of our study were consistent with the findings of these previous studies: reduced trunk muscle mass was associated with L4/5-disc space narrowing in both male and female; total, back, and psoas muscle mass, and fat/muscle ratio in female; and total and back muscle mass and fat/muscle ratio in male.
The method of measuring the muscle mass using CT can be divided according to the method used for determining the region of interest: the method of measuring the CSA of the outer edge of the muscle, and the area with the muscle-HU threshold. In this study, we used a computerized method to measure muscle mass using the HU threshold in CT; this method saves time and provides consistent results [31]. Different studies in the previous literature have used different ranges of interest for measuring muscle mass. Muscle mass areas found to be associated with LBP have included the multifidus [14], multifidus and erector spinae [13,32], paraspinal muscles (multifidus+erector spinae) [33,34], and paraspinal and psoas muscles [35]. These manual methods for demarcating the muscle mass area are time-consuming and rely on a technician with good anatomical knowledge. A simple measurement of the cross-sectional area along the boundaries of the muscle cannot exclude the amount of intramuscular fat. In our study, the total trunk muscle mass was automatically derived from a standard CT image using a thresholding technique [11] excluding fat infiltration, and the results showed that both the size of the back muscle as well as the whole trunk muscle mass are significantly related to L4/5-disc space narrowing.
As mentioned above, the CSA measurement method through the demarcation of muscle boundary cannot exclude the amount of fat infiltration in the muscle CSA. Fat infiltration into back muscles is associated with LBP, and variations in muscle composition have been observed in different regions of the trunk. Greater fat infiltration has been reported on the sides and at spinal levels adjacent to the disc herniation [7]. The paraspinal muscle area decreases with age, while the total amount of paraspinal fat increases with age [11]. Many studies that reported a decreased lumbar muscle mass with LBP, have assessed participants who are mostly young (in their 20s and 40s) [6]. In contrast, in studies that assessed older people in their 60s, no significant difference was observed in back muscle mass between the LBP and control groups [27]. The HU method enables the calculation of muscle mass that excludes intramuscular fat to be made.
In our study, LBP did not show a direct relationship with trunk muscle mass. The back muscle plays an important role in lumbar segmental stability, and if it atrophies, it may cause the recurrence of LBP [6]. However, some studies have shown that the relationship between back pain and trunk muscle mass was not significant. One study reported that, in 20 subjects without LBP, there was no significant difference in CSA with and without intervertebral disc degeneration of the lumbar spine [36]. Another study that followed 26 young healthy volunteers— male fighter pilots aged 20.6±0.6—for 5 years found no associations between muscle composition or CSA and LBP [37]. The difference in these study results may have been related to the areas in which the muscle mass was measured. Since we measured the trunk muscle mass at the L4 level, we did not measure the muscle mass of all the muscles surrounding the lumbar spine. Disturbed back muscle innervation and loss of muscular support lead to increased biomechanical strain. The fatto-muscle ratio can be considered an index that reflects both fat and muscle effects. In this study, the trunk fat/ muscle ratio was found to be significantly related to the narrowing of the L4/5-disc space and LBP, and this was observed in both male and female.
Farmers suffer from musculoskeletal pain, especially LBP. In this study, 32.2% of participants responded that they had seen a doctor at the hospital due to back pain during the last year. Little is known about the relationship between trunk muscle mass and LBP and disc space narrowing of the lumbar spine in farmers. Musculoskeletal pain in farmers is greatly affected by agricultural work. In particular, lifting, harvesting, and planting heavy things heavily affect the lumbar spine and the occurrence of LBP. Moreover, those with long-term farming experience of at least 30 years have a greater risk of LBP [1]. On the other hand, high levels of physical activity among farmers, particularly head-carrying among female, appear to be associated with higher trunk muscle endurance and strength [38]. In summary, we showed the relationship between trunk fat/muscle and LBP and lumbar disc space narrowing in farmers. These results are expected to be baseline data for future research on the prevention of lumbar diseases by increasing muscle strength, and on reducing back pain by reducing abdominal fat.
The main limitation of this study is its cross-sectional design. The outcomes of this study warrant further investigation and verification to determine whether a person’s trunk fat/muscle composition and fat-to-muscle ratio can predict future back pain and lumbar disc disorders. Moreover, we measured muscle and fat mass by one technician, and this did not provide measure-remeasure reliability. However, according to a previous study, muscle mass measurements using the HU threshold were reliable, regardless of the CT protocol used [31]. Also, we evaluated disc degeneration by X-ray, but did not evaluate disc damage by lumbar MRI or CT. The degree of back pain expressed as ODI may be measured in various ways depending on the evaluation time point. However, in the case of fat/muscle ratio or muscle mass, the degree of change is modest, so there seems to be a limitation in comparing the two evaluation indicators. Additionally, body fat percentage or disc degeneration are factors greatly affected by age. Some studies have shown that degeneration affects the fat infiltration of the back muscles, while others show that fat infiltration is more affected by age. Lastly, we conducted a study on farmers living in Gangwon-do, and, therefore, our findings may not be representative of the entire population of Korean farmers.
LBP and various fat indicators were shown to be significantly related. Trunk muscle mass was significantly related to lumbar disc degeneration, and the fat/muscle ratio was significantly related to lumbar disc degeneration as well as LBP in both male and female. It may be useful to investigate the effectiveness of using the fat/muscle ratio as an index for LBP.
ACKNOWLEDGMENTS
This study was supported by Research Grant for Center for Farmers’ Safety and Health at Kangwon National University Hospital from Korean Ministry of Agriculture, Food and Rural Affairs.
REFERENCES
1. Min D, Baek S, Park HW, Lee SA, Moon J, Yang JE, et al. Prevalence and characteristics of musculoskeletal pain in Korean farmers. Ann Rehabil Med. 2016; 40:1–13.

2. Walker-Bone K, Palmer KT. Musculoskeletal disorders in farmers and farm workers. Occup Med (Lond). 2002; 52:441–50.

3. Dianat I, Afshari D, Sarmasti N, Sangdeh MS, Azaddel R. Work posture, working conditions and musculoskeletal outcomes in agricultural workers. Int J Ind Ergon. 2020; 77:102941.

4. Kato S, Murakami H, Demura S, Yoshioka K, Shinmura K, Yokogawa N, et al. Abdominal trunk muscle weakness and its association with chronic low back pain and risk of falling in older women. BMC Musculoskelet Disord. 2019; 20:273.

5. Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, et al. Are the size and composition of the paraspinal muscles associated with low back pain?: a systematic review. Spine J. 2017; 17:1729–48.

6. Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. 2016; 19:E985–1000.
7. Fortin M, Lazary A, Varga PP, McCall I, Battie MC. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J. 2016; 25:1452–9.

8. Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O’Sullivan R, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in communitybased adults. Spine J. 2015; 15:1593–601.

9. Brady SRE, Urquhart DM, Hussain SM, Teichtahl A, Wang Y, Wluka AE, et al. High baseline fat mass, but not lean tissue mass, is associated with high intensity low back pain and disability in community-based adults. Arthritis Res Ther. 2019; 21:165.

10. Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol. 2010; 171:135–54.

11. McLoughlin RF, D’Arcy EM, Brittain MM, Fitzgerald O, Masterson JB. The significance of fat and muscle areas in the lumbar paraspinal space: a CT study. J Comput Assist Tomogr. 1994; 18:275–8.
12. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998; 85:115–22.

13. Gibbons LE, Videman T, Battie MC. Isokinetic and psychophysical lifting strength, static back muscle endurance, and magnetic resonance imaging of the paraspinal muscles as predictors of low back pain in men. Scand J Rehabil Med. 1997; 29:187–91.
14. Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000; 9:266–72.

15. Kamaz M, Kiresi D, Oguz H, Emlik D, Levendoglu F. CT measurement of trunk muscle areas in patients with chronic low back pain. Diagn Interv Radiol. 2007; 13:144–8.
16. Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol. 2014; 6:409–16.

17. Kim DY, Lee SH, Lee HY, Lee HJ, Chang SB, Chung SK, et al. Validation of the Korean version of the oswestry disability index. Spine (Phila Pa 1976). 2005; 30:E123–7.

18. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000; 25:2940–52.

19. Chun MY. Validity and reliability of Korean version of International Physical Activity Questionnaire short form in the elderly. Korean J Fam Med. 2012; 33:144–51.

20. Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976). 1994; 19:1371–80.

21. Hussain SM, Urquhart DM, Wang Y, Shaw JE, Magliano DJ, Wluka AE, et al. Fat mass and fat distribution are associated with low back pain intensity and disability: results from a cohort study. Arthritis Res Ther. 2017; 19:26.

22. Kim B, Oh S, Kim E. Abdominal fat accumulation as a potential risk factor for low back pain in adult men. Int J Appl Sports Sci. 2019; 31:25–31.

23. Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordonana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J. 2015; 15:1106–17.

24. Vismara L, Menegoni F, Zaina F, Galli M, Negrini S, Capodaglio P. Effect of obesity and low back pain on spinal mobility: a cross sectional study in women. J Neuroeng Rehabil. 2010; 7:3.

25. Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976). 2006; 31:2926–33.

26. Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine (Phila Pa 1976). 2007; 32:E598–602.

27. Yarjanian JA, Fetzer A, Yamakawa KS, Tong HC, Smuck M, Haig A. Correlation of paraspinal atrophy and denervation in back pain and spinal stenosis relative to asymptomatic controls. PM R. 2013; 5:39–44.
28. Fortin M, Yuan Y, Battie MC. Factors associated with paraspinal muscle asymmetry in size and composition in a general population sample of men. Phys Ther. 2013; 93:1540–50.

29. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976). 2008; 33:318–25.
30. Kang CH, Shin MJ, Kim SM, Lee SH, Lee CS. MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol. 2007; 62:479–86.

31. Kim DW, Ha J, Ko Y, Kim KW, Park T, Lee J, et al. Reliability of skeletal muscle area measurement on CT with different parameters: a phantom study. Korean J Radiol. 2021; 22:624–33.

32. Miki T, Naoki F, Takashima H, Takebayashi T. Associations between paraspinal muscle morphology, disc degeneration, and clinical features in patients with lumbar spinal stenosis. Prog Rehabil Med. 2020; 5:20200015.

33. Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine (Phila Pa 1976). 1993; 18:830–6.

34. Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine (Phila Pa 1976). 1994; 19:165–72.

35. Parkkola R, Kujala U, Rytokoski U. Response of the trunk muscles to training assessed by magnetic resonance imaging and muscle strength. Eur J Appl Physiol Occup Physiol. 1992; 65:383–7.

36. Gwak GT, Hwang UJ, Jung SH, Kim HA, Kim JH, Kwon OY. Comparison of MRI cross-sectional area and functions of core muscles among asymptomatic individuals with and without lumbar intervertebral disc degeneration. BMC Musculoskelet Disord. 2019; 20:576.

Fig. 1.
Computed tomography images at the mid-T4 level. (A) Ten consecutive image slices of 1-mm thickness were taken, covering a total thickness of 10 mm. (B) Automatically derived total muscle and fat volume using the threshold technique. (C) Manually subdivided back and psoas muscle masses and visceral fat.
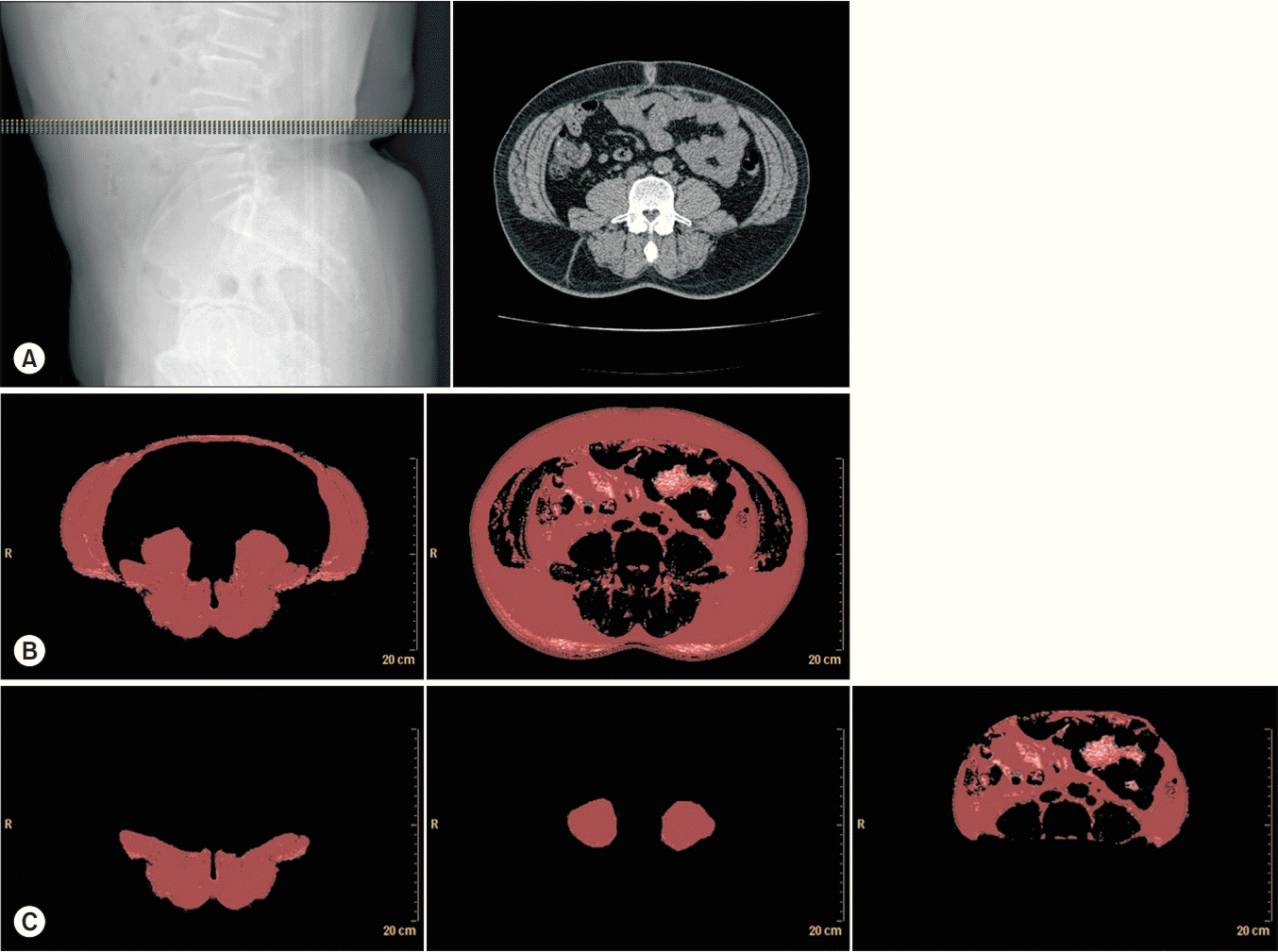
Table 1.
Characteristics of middle-aged farmers (n=463)
| Male (n=206) | Female (n=257) | Mean difference | p-valuea) | |
|---|---|---|---|---|
| Age (yr) | 56.57±5.93 | 55.81±5.78 | 0.75 | 0.168 |
| ODI score (0–100) | 9.37±11.02 | 13.43±13.52 | -4.06 | <0.001 |
| Height (cm) | 167.49±5.68 | 154.44±5.95 | 13.05 | <0.001 |
| Weight (kg) | 72.11±10.71 | 61.04±8.71 | 11.08 | <0.001 |
| BMI (kg/m2) | 25.65±3.11 | 25.57±3.09 | 0.08 | 0.782 |
| Waist circumference (cm) | 89.49±8.97 | 83.08±8.75 | 6.41 | <0.001 |
| Physical activity (MET-min/wk) | 5719.5±5394.7 | 5476.9±5701.7 | 242.58 | 0.641 |
| Total fat mass (cm3) | 272.54±98.03 | 288.3±87.02 | -15.77 | 0.068 |
| Visceral fat mass (cm3) | 115.22±49.44 | 92.01±39.08 | 23.21 | <0.001 |
| Subcutaneous fat mass (cm3) | 157.31±62.06 | 196.29±62.93 | -38.98 | <0.001 |
| Total muscle mass (cm3) | 158.4±22.7 | 110.34±14.7 | 48.06 | <0.001 |
| Back muscle mass (cm3) | 66.9±9.56 | 51.6±7.72 | 15.3 | <0.001 |
| Psoas muscle mass (cm3) | 26.89±14.49 | 14.93±4.67 | 11.96 | <0.001 |
| Abdominal muscle mass (cm3) | 64.61±18.9 | 43.81±8.17 | 20.8 | <0.001 |
| Trunk fat/muscle ratio | 1.72±0.59 | 2.63±0.76 | -0.91 | <0.001 |
| Farming types | ||||
| Rice farming | 24 (11.7) | 7 (2.7) | <0.001 | |
| Dry fields farming | 60 (29.1) | 114 (44.4) | ||
| Greenhouses farming | 94 (45.6) | 116 (45.1) | ||
| Orchards farming | 28 (13.6) | 20 (7.8) | ||
| L3/4-disc space narrowing | ||||
| Grade 0 | 165 (80.1) | 192 (74.7) | 0.488b) | |
| Grade 1 | 26 (12.6) | 35 (13.6) | ||
| Grade 2 | 9 (4.4) | 14 (5.4) | ||
| Grade 3 | 4 (1.9) | 11 (4.3) | ||
| Grade 4 | 2 (1) | 5 (1.9) | ||
| L4/5-disc space narrowing | ||||
| Grade 0 | 122 (59.2) | 133 (51.8) | 0.05b) | |
| Grade 1 | 46 (22.3) | 56 (21.8) | ||
| Grade 2 | 24 (11.7) | 27 (10.5) | ||
| Grade 3 | 10 (4.9) | 27 (10.5) | ||
| Grade 4 | 4 (1.9) | 14 (5.4) | ||
| L5/S1-disc space narrowing | ||||
| Grade 0 | 76 (36.9) | 60 (23.3) | 0.002b) | |
| Grade 1 | 66 (32) | 72 (28) | ||
| Grade 2 | 24 (11.7) | 46 (17.9) | ||
| Grade 3 | 26 (12.6) | 50 (19.5) | ||
| Grade 4 | 14 (6.8) | 29 (11.3) | ||
| Spondylolisthesis | ||||
| No | 187 (90.8) | 223 (86.8) | 0.178b) | |
| Yes | 19 (9.2) | 34 (13.2) |
Table 2.
Pearson correlation coefficients (r) for anthropometric measurements and trunk fat/muscle mass with the OSI score
|
Male (n=206) |
Female (n=257) |
|||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Height (cm) | -0.054 | 0.444 | 0.082 | 0.19 |
| Weight (kg) | 0.037 | 0.593 | 0.118 | 0.059 |
| BMI (kg/m2) | 0.076 | 0.275 | 0.079 | 0.207 |
| Waist circumference (cm) | 0.06 | 0.392 | 0.178 | 0.004** |
| Total fat mass | 0.099 | 0.158 | 0.124 | 0.048* |
| Visceral fat mass | 0.104 | 0.139 | 0.151 | 0.015* |
| Subcutaneous fat mass | 0.073 | 0.294 | 0.077 | 0.218 |
| Total muscle mass | 0.004 | 0.951 | 0.009 | 0.887 |
| Back muscle mass | -0.036 | 0.604 | -0.006 | 0.925 |
| Psoas muscle mass | 0.009 | 0.902 | -0.079 | 0.207 |
| Abdominal muscle mass | 0.017 | 0.81 | 0.067 | 0.286 |
| Trunk fat/muscle ratio | 0.122 | 0.08 | 0.123 | 0.049* |
Table 3.
Pearson correlation coefficients (r) for anthropometric measurements and trunk fat/muscle mass with the physical activity (MET-min/wk)
|
Male (n=206) |
Female (n=257) |
|||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Height (cm) | -0.055 | 0.436 | 0.055 | 0.376 |
| Weight (kg) | 0.023 | 0.738 | 0.066 | 0.289 |
| BMI (kg/m2) | 0.056 | 0.428 | 0.037 | 0.552 |
| Waist circumference (cm) | -0.005 | 0.945 | 0.037 | 0.551 |
| Total fat mass | -0.071 | 0.314 | 0.035 | 0.575 |
| Visceral fat mass | -0.09 | 0.198 | -0.038 | 0.543 |
| Subcutaneous fat mass | -0.04 | 0.571 | 0.072 | 0.249 |
| Total muscle mass | 0.02 | 0.773 | 0.152 | 0.015* |
| Back muscle mass | 0.049 | 0.48 | 0.167 | 0.007** |
| Psoas muscle mass | -0.062 | 0.377 | 0.011 | 0.867 |
| Abdominal muscle mass | 0.047 | 0.504 | 0.11 | 0.079 |
| Trunk fat/muscle ratio | -0.077 | 0.272 | -0.029 | 0.647 |
Table 4.
Spearman rho of anthropometric measurements and trunk fat/muscle mass with grades of L3/4-, L4/5-, and L5/S1 disc space narrowing and spondylolisthesis
|
L3/4 |
L4/5 |
L5/S1 |
Spondylolisthesis |
|||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Height (cm) | -0.008 | -0.058 | 0.058 | -0.081 | 0.15* | 0.16* | -0.058 | -0.098 |
| Weight (kg) | 0.088 | -0.004 | 0.043 | 0.059 | 0.085 | 0.129* | 0.101 | 0.031 |
| BMI (kg/m2) | 0.106 | 0.048 | 0.029 | 0.112 | 0.017 | 0.044 | 0.144* | 0.11 |
| Waist circumference (cm) | 0.081 | 0.042 | 0.045 | 0.136 | -0.001 | 0.093 | 0.143* | 0.066 |
| Total fat mass | 0.099 | 0.039 | 0.087 | 0.134* | 0.132 | 0.101 | 0.134 | 0.075 |
| Visceral fat mass | -0.004 | 0.125* | 0.008 | 0.127* | 0.085 | 0.167** | 0.045 | 0.094 |
| Subcutaneous fat mass | 0.126 | -0.028 | 0.103 | 0.108 | 0.109 | 0.03 | 0.163* | 0.039 |
| Total muscle mass | 0.006 | -0.138* | -0.168* | -0.252*** | -0.029 | -0.021 | -0.001 | 0.006 |
| Back muscle mass | -0.146* | -0.227*** | -0.271*** | -0.314*** | -0.144* | -0.073 | 0.053 | -0.013 |
| Psoas muscle mass | 0.061 | -0.076 | -0.166* | -0.199** | -0.076 | -0.133* | -0.03 | -0.102 |
| Abdominal muscle mass | 0.08 | -0.012 | -0.033 | -0.074 | 0.071 | 0.099 | -0.039 | 0.048 |
| Trunk fat/muscle ratio | 0.086 | 0.116 | 0.166* | 0.267*** | 0.127 | 0.107 | 0.139* | 0.068 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download