1. Beny K, Piriou V, Dussart C, Hénaine R, Aulagner G, Armoiry X; experts du NMBA pharmacoepidemiology group. [Impact of sugammadex on neuromuscular blocking agents use: a multicentric, pharmaco-epidemiologic study in French university hospitals and military hospitals]. Ann Fr Anesth Reanim. 2013; 32:838–43. French.
2. Pavoni V, Gianesello L, De Scisciolo G, Provvedi E, Horton D, Barbagli R, et al. Reversal of profound and "deep" residual rocuronium-induced neuromuscular blockade by sugammadex: a neurophysiological study. Minerva Anestesiol. 2012; 78:542–9.
3. Woo T, Kim KS, Shim YH, Kim MK, Yoon SM, Lim YJ, et al. Sugammadex versus neostigmine reversal of moderate rocuronium-induced neuromuscular blockade in Korean patients. Korean J Anesthesiol. 2013; 65:501–7.

4. Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, et al. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl. 2002; 41:266–70.

5. Morris RB, Cronnelly R, Miller RD, Stanski DR, Fahey MR. Pharmacokinetics of edrophonium and neostigmine when antagonizing d-tubocurarine neuromuscular blockade in man. Anesthesiology. 1981; 54:399–401.

6. Donati F, McCarroll SM, Antzaka C, McCready D, Bevan DR. Dose-response curves for edrophonium, neostigmine, and pyridostigmine after pancuronium and d-tubocurarine. Anesthesiology. 1987; 66:471–6.

7. Caldwell JE. Clinical limitations of acetylcholinesterase antagonists. J Crit Care. 2009; 24:21–8.

8. Zwiers A, van den Heuvel M, Smeets J, Rutherford S. Assessment of the potential for displacement interactions with sugammadex: a pharmacokinetic-pharmacodynamic modelling approach. Clin Drug Investig. 2011; 31:101–11.
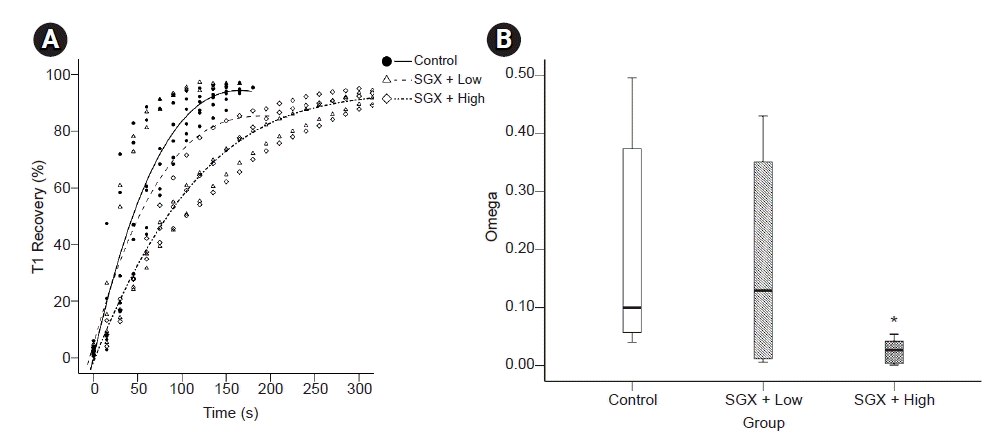
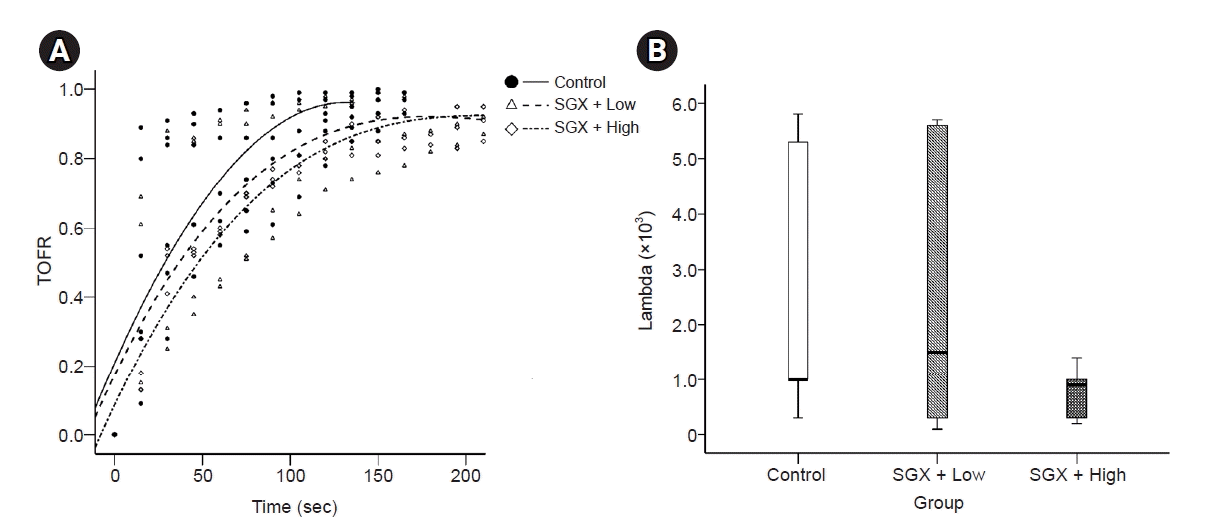
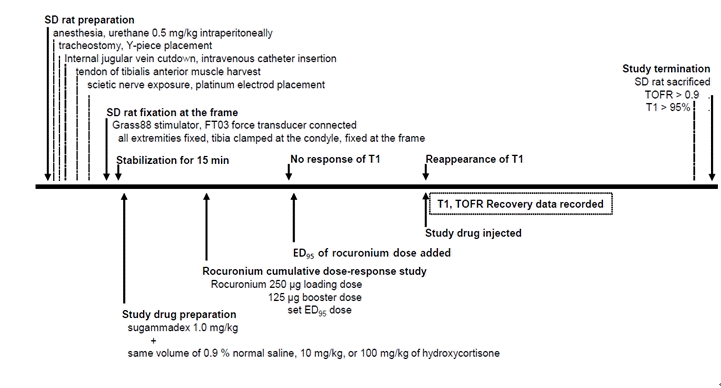
9. Choi H, Park SY, Kim YB, In J, Yang HS, Lee JS, et al. Effects of dexamethasone and hydrocortisone on rocuroniuminduced neuromuscular blockade and reversal by sugammadex in phrenic nerve-hemidiaphragm rat model. Korean J Anesthesiol. 2019; 72:366–74.

10. Choi JM, Kim HJ, Choi HR, Kim YB, Bae HJ, Yang HS. Remifentanil does not inhibit sugammadex reversal after rocuronium-induced neuromuscular block in the isolated hemidiaphragm of the rat: an ex vivo study. J Anesth. 2019; 33:642–6.

11. Kim YB, Yang HS, Kim HJ, Choi HR, In J, Yoon SY, et al. Effects of neuromuscular presynaptic muscarinic M1 receptor blockade on rocuronium-induced neuromuscular blockade in immobilized tibialis anterior muscles. Clin Exp Pharmacol Physiol. 2018; 45:1309–16.

12. Kim YB, Lee S, Choi HR, In J, Chang YJ, Kim HJ, et al. Effects of adenosine receptor agonist on the rocuroniuminduced neuromuscular block and sugammadex-induced recovery. Korean J Anesthesiol. 2018; 71:476–82.

13. Keegan MT. Endocrine pharmacology. Pharmacology and physiology for anesthesia: foundations and clinical application. In : Hemmings HC, Egan TD, editors. Philadelphia (PA): Elsevier;2019. p. 708–31.
14. Liu MM, Reidy AB, Saatee S, Collard CD. Perioperative steroid management: approaches based on current evidence. Anesthesiology. 2017; 127:166–72.
15. Woodcock T, Barker P, Daniel S, Fletcher S, Wass JAH, Tomlinson JW, et al. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia. 2020; 75:654–63.

16. Seo KH. Perioperative glucocorticoid management based on current evidence. Anesth Pain Med (Seoul). 2021; 16:8–15.

17. Kam PJ, Heuvel MW, Grobara P, Zwiers A, Jadoul JL, Clerck Ed, et al. Flucloxacillin and diclofenac do not cause recurrence of neuromuscular blockade after reversal with sugammadex. Clin Drug Investig. 2012; 32:203–12.

18. Rezonja K, Sostaric M, Vidmar G, Mars T. Dexamethasone produces dose-dependent inhibition of sugammadex reversal in in vitro innervated primary human muscle cells. Anesth Analg. 2014; 118:755–63.

19. Gulec E, Biricik E, Turktan M, Hatipoglu Z, Unlugenc H. The effect of intravenous dexamethasone on sugammadex reversal time in children undergoing adenotonsillectomy. Anesth Analg. 2016; 122:1147–52.

20. Chen W, Chang CE, Gilson MK. Calculation of cyclodextrin binding affinities: energy, entropy, and implications for drug design. Biophys J. 2004; 87:3035–49.

21. Chodera JD, Mobley DL. Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu Rev Biophys. 2013; 42:121–42.

22. Fuchs-Buder T, Meistelman C, Raft J. Sugammadex: clinical development and practical use. Korean J Anesthesiol. 2013; 65:495–500.

23. Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008; 109:816–24.
24. Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010; 112:1013–22.

25. Kotake Y, Ochiai R, Suzuki T, Ogawa S, Takagi S, Ozaki M, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013; 117:345–51.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download