Abstract
Aspiration catheters are widely used in mechanical thrombectomy procedures to treat acute ischemic stroke due to large vessel occlusion. The key performance requirements for aspiration catheters are ease of navigation and effective aspiration. In this work, we review the clinical experience and in vitro studies of REACT aspiration catheters (Medtronic, Minneapolis, MN, USA). In vitro experiments showed that REACT catheters exhibit solid performance in navigation and aspiration. Previous studies reported that the recanalization capacity of the aspiration catheters can be influenced by the devices’ inner diameter and tip distensibility, the catheter-to-vessel diameter ratio, the negative pressure delivered by the vacuum generator, the cyclical aspiration mode, the proximal flow arrest, and the angle of interaction between catheter and clot. REACT catheters can be navigated through the vasculature without any support from a microcatheter/ microwire in favorable anatomical configurations. In challenging situations, mostly encountered when crossing the ophthalmic segment of the internal carotid artery, the use of the stentriever anchoring technique or delivery assist catheter can facilitate the navigation. Three clinical studies reporting on 299 patients who underwent mechanical thrombectomy with REACT catheters were included in this review. Successful recanalization (modified treatment in cerebral ischemia score 2b–3) was achieved in 89–96% of cases, no procedural complications related to REACT catheters were reported, and functional independence (modified Rankin Scale 0–2) at 90-days was 24–36%. In vitro experimental evaluations and clinical studies support the safety and effectiveness of the REACT catheters.
Mechanical thrombectomy has been established as the standard treatment for acute ischemic stroke due to large vessel occlusion [1-4]. Over the last decade, a variety of new devices and techniques have been developed to reduce the recanalization time and improve clinical outcomes [5,6].
Aspiration catheters, also known as intermediate or distal access catheters, are frequently used in mechanical thrombectomy procedures to perform direct contact aspiration (a direct aspiration first pass technique, ADAPT) or a combined technique with a stentriever. In both technical approaches, aspiration catheters are navigated through the vascular anatomy to reach the occlusion site. The proximal end of the catheter is then connected to a syringe or aspiration pump that generates negative pressure to engage or ingest the thrombus. Therefore, the key performance requirements for distal access catheters are ease of navigation and effective aspiration.
The main components of navigability are trackability, which is the ability to advance over a rail to reach the target site, and pushability, which is the ability to transmit a push force from the proximal to the distal end of the device. Moreover, since the vascular anatomy can be highly tortuous, the catheter shaft should be resistant to kink when crossing tight turns. Device manufacturers achieve these characteristics by adjusting the design of the 4 elements that constitute the catheters: inner liner, reinforcement, outer jacket, and hydrophilic coating (Fig. 1). In broad terms, to enhance the trackability, the liner needs to be made of a low-frictional material such as polytetrafluoroethylene, commonly known as Teflon (PTFE); to improve the pushability, the reinforcement requires a braided structure that favors force transmission along the catheter shaft; and to increase the kink and ovalization resistance, the reinforcement needs a coil structure that provides flexibility and hoop strength. Additionally, the outer jacket made of polymers of variable composition allows further adjustment of catheter flexibility, and the hydrophilic coating on the polymer jacket can increase the lubricity of the device, ie, reduce the friction between catheter and vessel walls, during navigation.
The aspiration performance of distal access catheters can be evaluated by aspiration flow rate when the catheter tip is not in contact or immediately proximal to the clot, or aspiration force when the thrombus is occluding the catheter tip. In either case, the aspiration capacity increases with the catheter’s inner diameter and the vacuum generator’s power. Furthermore, previous studies reported that maximizing the catheter-to-vessel diameter ratio allowed to achieve higher reperfusion rates [7], as the presence of the catheter in the target vessel induced a local flow arrest [8]. Thus, there is an upward tendency in the use of larger-bore aspiration catheters.
REACT 68 and REACT 71 (Medtronic, Minneapolis, MN, USA) are large-bore aspiration catheters that feature a PTFE liner, nitinol reinforcement with a hybrid configuration combined coil and braid, flexible polymer jacket, and hydrophilic coating on the devices’ distal end. In this work, we review the clinical experience and in vitro studies of REACT catheters, and discuss technical tips to optimize mechanical thrombectomy procedures.
Three clinical studies reporting on 299 patients who underwent mechanical thrombectomy with REACT catheters were included in this review [9-11]. Demographics, procedural data, and outcomes are presented in Table 1. Study variables include median age, sex, occlusion site, procedural technique, device, mean/median number of passes, mean/median procedural time, successful recanalization according to modified treatment in cerebral ischemia score (mTICI 2b–3, >50% reperfusion of the target vessel) after the first pass, complete recanalization (mTICI 2c–3) within a single attempt (first-pass effect [FPE]), overall procedural success (mTICI 2b–3 and 2c–3), rate of complications, and functional outcome according to modified Rankin Scale (mRS 0–2) at 90 days.
Requena et al. [9] presented a prospective study including 102 consecutive adult patients. Stent-retriever assisted vacuum-locked extraction (SAVE) (simultaneous retrieval of stentriever and distal access catheter under continuous aspiration) or Solumbra (re-sheathing of stentriever under continuous aspiration without retracting the catheter) techniques were performed using REACT catheters (REACT 68 or REACT 71) in combination with different stentrievers. For the distal access, the interventionalists utilized the stentriever anchoring technique. This navigation approach consists in, first, placing a long 6F sheath in the internal carotid artery or vertebral artery through a percutaneous transfemoral or transradial access. Next, a REACT catheter and a 0.021 inch microcatheter with a 0.014 inch microwire are introduced into the coaxial system. The microcatheter is then tracked further over the microwire to cross the occlusion site. Lastly, the stentriever is deployed over the clot and used as an anchor to apply tension over the wire and navigate the aspiration catheter over the rail to the proximal interface of the thrombus. In this study, successful recanalization (mTICI 2b–3) was achieved in 89% of patients with a complete recanalization (mTICI 2c–3) rate of 65%. Successful recanalization was achieved in a single pass in 54% of patients and FPE in 36%. The median procedural time from arterial puncture to recanalization was 40 (26–53) minutes, and the median number of passes was 2 (1–3). No procedural complications related to REACT catheters were reported. Functional independence (mRS 0–2) at 90 days was 36%.
Raymond et al. [10] attempted the sofia non-wire advancement technike (SNAKE) technique, i.e., navigation without requiring microcatheter or microwire support, in 47 thrombectomies performed with REACT 68. The unsupported catheter successfully reached the proximal interface of the clot in 21% of attempts. Microcatheter or wire support was required in 56% of cases and the stentriever anchoring technique was employed in 23% of cases. This retrospective observational study reported that successful recanalization (mTICI 2b–3) using either ADAPT or aspiration combined with a stentriever was achieved in 95.7% of patients. The median procedural time from arterial puncture to recanalization was 22 (16.5–43) minutes, and the median number of passes was 2 (1–3). No procedural complications related to REACT 68 were encountered. Functional independence (mRS 0–2) at 90 days was 23.5%.
Gross et al. [11] reported 150 thrombectomies performed with REACT 71. The interventionalists delivered the aspiration catheter to the occlusion site over a 0.025 or 0.027 inch microcatheter, or using the stentriever anchoring technique. In this prospective study, successful recanalization (mTICI 2b–3) using either ADAPT or a combined technique was achieved in 95% of patients with a complete recanalization (mTICI 2c–3) rate of 39%. Successful recanalization was achieved in a single pass in 51% of patients and FPE in 26%. The mean procedure duration was 35 minutes, and the mean number of total passes was 2.2. No procedural complications related to REACT 71 were reported. Functional independence (mRS 0–2) at 90 days was 34%.
On theoretical grounds, the aspiration performance of a catheter is dictated by the Poiseuille’s law, Equation 1:
Where Q=aspiration flow rate, P=negative pressure generated by syringe or aspiration pump, D=catheter’s inner diameter, µ=blood viscosity, and l=catheter length. However, to maximize the effectiveness of aspiration [12] and to avoid arterial collapse that may lead to vasospasm [13], previous studies remarked on the importance of positioning the catheter tip en face to the clot before initiating aspiration. In this case, where the clot is corked into the catheter tip and there is no backflow (Q=0) through the device, the aspiration force is governed by Equation 2:
Where F=aspiration force, P=negative pressure generated by syringe or aspiration pump, and D=catheter’s inner diameter. In line with the theoretical equations, technical in vitro evaluations by several authors suggest that the aspiration performance (both in terms of force and flow rate) of distal access catheters is highly dependent on the labeled inner diameter specified by device manufacturers [8,14-19]. However, this rationale is only applicable when the thrombus is softer than the catheter tip and only the clot experiences strain (e.g., erythrocyte-rich thrombus) [20]. In reality, the distal edge above the radiopaque marker band of the catheters is the softest part of the device, commonly constituted by the liner and/or the polymeric jacket layer. During aspiration thrombectomy, firm clots (e.g., fibrin/platelet-rich thrombus) [20], that cannot be completely ingested due to their size and stiffness, may be capable of inducing catheter tip expansion, which implies that the inner diameter during aspiration can differ from the labeled one.
A recent in vitro study [21] refers to the capability of inner diameter expansion as catheter tip distensibility. This work differentiates labeled inner diameter from effective inner diameter, which takes into consideration the influence of clot biomechanics on the catheter tip. The experiments measured the aspiration forces of commercially available catheters and showed that, when aspirating firm clots, all devices presented a certain degree of tip distensibility (15–25% expansion of inner diameter) that allowed them to deliver aspiration forces 60–99% higher than theoretical forces according to Equation 2. Among all devices evaluated, REACT 71 presented the highest tip distensibility, largest effective inner diameter and, consequently, the highest aspiration force despite not being the largest-bore catheter included in the study. Comparison of aspiration performance among various catheters is summarized in Table 2.
In benchtop testing platforms, the proximal end of the aspiration catheter is attached to a motorized force sensor that advances the device at a constant velocity to navigate it through a preset trajectory. Motor-driven dynamometers substitute operators’ force discrimination ability and allow for a more objective, consistent, and reproducible assessment of devices’ navigability. Track forces required to traverse tortuous paths are recorded as the quantitative metric of navigability. As higher forces exerted against the vascular walls may cause endothelial damage, lower track forces are deemed as an indicator of better navigability.
An experimental evaluation assessed the navigability of various aspiration catheters when advancing over a microcatheter/microwire support system [22]. This study reported that REACT 71 was one of the devices that required less track force to navigate through a sinusoidal vascular anatomy made of glass.
Similarly, a recent in vitro study [23] compared the SNAKE navigation approach versus the concomitant coaxial use of microwire/microcatheter and the stentriever anchoring technique. This study utilized a glass-made model that reproduced a simplified version of the vascular anatomy from the right common carotid artery to the M1 segment of the middle cerebral artery. In comparison to the SNAKE technique, track forces were reduced by about 63% and 77% when using microcatheter/microwire and the stentriever anchoring technique, respectively. This may have been due to the presence of the microcatheter/stentriever rail that aligned the catheters’ trajectory with the vessel centerline, which minimized the occurrence rate of ledge effect, ie, the inability to advance the distal access catheter past the ophthalmic segment of the carotid siphon because of the engagement between the catheter tip and ophthalmic artery origin [24]. This work showed up to 79% variability in catheters’ navigation performance. Among all devices evaluated, when traversing the ophthalmic segment, REACT 71 was one of the catheters that exhibited smoother navigation with microcatheter/microwire and stentriever anchoring techniques. This was probably due to the hybrid coil and braid reinforcement made of nitinol wires in the REACT catheters’ design, the braid structure enhanced the pushability, the coil provided kink and ovalization resistance, and nitinol wires added flexibility and durability to the device. Comparison of navigability among various aspiration catheters is summarized in Table 2.
The ultimate goal of mechanical thrombectomy is the achievement of complete reperfusion in a single attempt, which has been reported as a strong predictor of better clinical outcomes. Nevertheless, FPE was reported to have occurred at a rate of 25–40% of cases in clinical studies. To increase the chances for FPE, it is essential to identify and optimize those parameters affecting the performance of different thrombectomy devices. In this article, we reviewed the key performance requirements for aspiration catheters.
Aspiration catheters’ inner diameter, thrombus stiffness and catheter tip distensibility, and catheter-to-vessel diameter ratio are variables that can influence the aspiration performance of the devices. Other procedural-related factors that can vary the recanalization capacity of aspiration catheters are:
• The negative pressure generated by the aspiration pump/syringe: both aspiration flow rate and force depend on the vacuum pressure. Commercially available pumps do not necessarily generate more vacuum pressure than syringes. Under flow restriction conditions (clot completely occluding the catheter tip), 60 cc syringes showed slightly higher vacuum pressure than aspiration pumps, probably due to resistive components in the pumps’ system, such as the silicone tubing and on-off switch; under partial backflow conditions (incomplete apposition of the catheter tip against clot), syringes outperformed pumps until the entire syringe was filled with fluid, after that, the syringes’ suction power dropped to zero while the pumps’ remained constant due to the greater volume of the canister [25].
• Static versus cyclical aspiration mode: the constant pressure delivered by conventional aspiration syringes and pumps is referred to as the static or continuous uniform aspiration. Increasing aspiration cycle frequency (pressure oscillations per second, Hz) can improve the efficacy of clot removal [26], as cyclical aspiration may be able to induce larger strain on the clots [27]. Clinical studies reported that cyclical aspiration, also known as pulsatile or intermittent aspiration, could be more effective than continuous uniform aspiration to achieve FPE [28,29].
• The utilization of balloon guide catheter: the combined effect of proximal flow arrest and distal aspiration can reduce the number of passes, time from puncture to recanalization, and periprocedural embolization [30,31]. Three force components act on a lodged thrombus and hinder clot removal: the impaction force exerted by the systemic pressure on the proximal face of the clot, the adhesion force, and the friction force between the thrombus and vessel wall [32]. Kang et al. [30] suggested that the use of balloon guide catheters mitigates the impaction force, and thus, diminishes the aspiration/retrieval force required for clot extraction.
• The interaction between clot and catheter: as reported by Bernava et al. [12], “the angle of interaction between the aspiration catheter and the clot of ≥125.5° was significantly associated with successful clot removal” and the rate of FPE was higher when the angle was approximately 180°. This finding highlights the importance of positioning the catheter tip against the proximal interface of the thrombus.
The ledge effect has been reported as a predictor of unsuccessful recanalization [33]. In vitro and clinical studies have shown that the stentriever anchoring technique was an effective alternative when the SNAKE technique or microcatheter/microwire support system failed to deliver aspiration catheters past the ophthalmic segment of the carotid siphon. The effectiveness of the stentriever anchoring technique can be attributed to the “rail” effect of applying tension on the wire after deploying the stentriever. The pulling force to generate tension should not exceed the radial force of the stentriever over the vascular wall, otherwise, the stentriever would be dislodged. Then, when the tensioned stentriever wire acts as a rail, the trajectory of the aspiration catheter is driven to the inner curve of the ophthalmic segment, which reduces the occurrence rate of the ledge effect.
However, the stentriever anchoring technique implies crossing the occlusion site with the microcatheter/microwire, which may induce thrombus fragmentation and distal embolization. To minimize the interaction with the thrombus, an emergent device class named delivery assist catheter has been evaluated as an alternative to existing approaches [24,34]. The operating principle behind the delivery assist catheter is the reduction of space between the microcatheter and aspiration catheter. Previous studies have shown that a 0.035 inch rather than 0.027 inch microcatheter reduces the frequency of the ledge effect [35]. In other words, delivery assist catheters are larger-caliber microcatheters that function as a more robust rail to keep aspiration catheters from 0.068 inch to 0.072 inch away from the ophthalmic artery origin. A recent in vitro study [24] reported that TENZING 7 (Route 92 Medical, San Mateo, CA, USA), a delivery assist catheter, was capable of delivering aspiration catheters to the proximal M1 in half the time required for the microcatheter/microwire support system. The use of delivery assist catheters could be complementary to further reduce procedural times and refine ADAPT.
In vitro studies support the effectiveness of REACT catheters, as both catheters exhibited solid performance in aspiration and navigability evaluations. Clinical studies using REACT aspiration catheters reported high rates of successful recanalization in patients with acute ischemic stroke due to large vessel occlusion. The rates of procedural success and recanalization at the first attempt, along with the null incidence of complications related to REACT catheters, demonstrate the safety and effectiveness of the devices.
Notes
Ethics Statement
Not applicable. The consent for publication is not required as this article does not include any images or information that may identify the person.
REFERENCES
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.

2. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019; 4:6–12.

3. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, HERMES collaborators, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.

4. Nogueira RG, Frei D, Kirmani JF, Zaidat O, Lopes D, Turk AS 3rd, Penumbra Separator 3D Investigators, et al. Safety and efficacy of a 3-dimensional stent retriever with aspiration-based thrombectomy vs aspiration-based thrombectomy alone in acute ischemic stroke intervention: a randomized clinical trial. JAMA Neurol. 2018; 75:304–311.

5. Spiotta AM, Chaudry MI, Hui FK, Turner RD, Kellogg RT, Turk AS. Evolution of thrombectomy approaches and devices for acute stroke: a technical review. J Neurointerv Surg. 2015; 7:2–7.

6. Bhogal P, Andersson T, Maus V, Mpotsaris A, Yeo L. Mechanical thrombectomy-a brief review of a revolutionary new treatment for thromboembolic stroke. Clin Neuroradiol. 2018; 28:313–326.

7. Kyselyova AA, Fiehler J, Leischner H, Flottmann F, Buhk JH, Frölich AM. Vessel diameter and catheter-to-vessel ratio affect the success rate of clot aspiration. J Neurointerv Surg. 2021; 13:605–608.

8. Nogueira RG, Ryan D, Mullins L, Thornton J, Fitzgerald S. Maximizing the catheter-to-vessel size optimizes distal flow control resulting in improved revascularization in vitro for aspiration thrombectomy. J Neurointerv Surg. 2022; 14:184–188.

9. Requena M, Piñana C, Olive-Gadea M, Hernández D, Boned S, De Dios M, et al. Combined technique as first approach in mechanical thrombectomy: efficacy and safety of REACT catheter combined with stent retriever. [published online ahead of print May 2, 2022]. Interv Neuroradiol. 2022.

10. Raymond SB, Nasir-Moin M, Koch MJ, Rabinov JD, Leslie-Mazwi T, Patel AB. Initial experience with React 68 aspiration catheter. Interv Neuroradiol. 2020; 26:358–363.

11. Gross BA, Hudson JS, Tonetti DA, Desai SM, Lang MJ, Jadhav AP, et al. Bigger is still better: a step forward in reperfusion with React 71. Neurosurgery. 2021; 88:758–762.
12. Bernava G, Rosi A, Boto J, Brina O, Kulcsar Z, Czarnetzki C, et al. Direct thromboaspiration efficacy for mechanical thrombectomy is related to the angle of interaction between the aspiration catheter and the clot. J Neurointerv Surg. 2020; 12:396–400.

13. Liu Y, Gebrezgiabhier D, Zheng Y, Shih AJ, Chaudhary N, Pandey AS, et al. Arterial collapse during thrombectomy for stroke: clinical evidence and experimental findings in human brains and in vivo models. AJNR Am J Neuroradiol. 2022; 43:251–257.

14. Froehler MT. Comparison of vacuum pressures and forces generated by different catheters and pumps for aspiration thrombectomy in acute ischemic stroke. Interv Neurol. 2017; 6:199–206.

15. Yaeger K, Iserson A, Singh P, Wolf J, Vidal E, Oxley T, et al. A technical comparison of thrombectomy vacuum aspiration systems. J Neurointerv Surg. 2020; 12:72–76.

16. Fernandez-Sanchez D, Garcia-Sabido D, Jovin TG, Villanova H, Andersson T, Nogueira RG, et al. Suction force rather than aspiration flow correlates with recanalization in hard clots: an in vitro study model. J Neurointerv Surg. 2021; 13:1157–1161.

17. Nikoubashman O, Wischer D, Hennemann HM, Büsen M, Brockmann C, Wiesmann M. Under pressure: comparison of aspiration techniques for endovascular mechanical thrombectomy. AJNR Am J Neuroradiol. 2018; 39:905–909.

18. Hu YC, Stiefel MF. Force and aspiration analysis of the ADAPT technique in acute ischemic stroke treatment. J Neurointerv Surg. 2016; 8:244–246.

19. Boisseau W, Escalard S, Fahed R, Lapergue B, Smajda S, Maier B, et al. Direct aspiration stroke thrombectomy: a comprehensive review. J Neurointerv Surg. 2020; 12:1099–1106.

20. Chueh JY, Wakhloo AK, Hendricks GH, Silva CF, Weaver JP, Gounis MJ. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol. 2011; 32:1237–1244.

21. Li J, Castaño O, Tomasello A, de Dios Lascuevas M, Canals P, Engel E, et al. Catheter tip distensibility substantially influences the aspiration force of thrombectomy devices. J Neurointerv Surg. 2022; 14:neurintsurg-2021-017487.

22. Reymond P, Brina O, Girdhar G, Bolanos O, Lovblad KO, Machi P. Experimental evaluation of the performance of large bore aspiration catheters. [published online ahead of print Feb 19, 2022]. J Neuroradiol. 2022.

23. Li J, Tomasello A, Requena M, Canals P, Tiberi R, Galve I, et al. Trackability of distal access catheters: an in vitro quantitative evaluation of navigation strategies. [published online ahead of print Apr 21, 2022]. J Neurointerv Surg. 2022.

24. Frölich AM, Kim W, Stribrny K, Jansen O, Möhlenbruch M, Szikora I, et al. The novel Tenzing 7 delivery catheter designed to deliver intermediate catheters to the face of embolus without crossing: clinical performance predicted in anatomically challenging model. J Neurointerv Surg. 2021; 13:722–726.

25. Gross BA, Jadhav AP, Jovin TG, Jankowitz BT. Dump the pump: manual aspiration thrombectomy (MAT) with a syringe is technically effective, expeditious, and cost-efficient. J Neurointerv Surg. 2018; 10:354–357.

26. Simon S, Grey CP, Massenzo T, Simpson DG, Longest PW. Exploring the efficacy of cyclic vs static aspiration in a cerebral thrombectomy model: an initial proof of concept study. J Neurointerv Surg. 2014; 6:677–683.

27. Marosfoi M, Strittmatter L, Arslanian R, Raskett C, Gounis M, Puri A, et al. E-112 How do clots respond to direct aspiration during interventional treatment of acute ischemic stroke. J Neurointerv Surg. 2019; 11(Suppl 1):A109–A110.
28. Arslanian RA, Marosfoi M, Caroff J, King RM, Raskett C, Puri AS, et al. Complete clot ingestion with cyclical ADAPT increases firstpass recanalization and reduces distal embolization. J Neurointerv Surg. 2019; 11:931–936.

29. Kalousek V, Yoo AJ, Sheth SA, Janardhan V, Mamic J, Janardhan V. Cyclical aspiration using a novel mechanical thrombectomy device is associated with a high TICI 3 first pass effect in large-vessel strokes. J Neuroimaging. 2021; 31:912–924.

30. Kang DH, Kim BM, Heo JH, Nam HS, Kim YD, Hwang YH, et al. Effect of balloon guide catheter utilization on contact aspiration thrombectomy. J Neurosurg. 2018; 131:1494–1500.

31. Stampfl S, Pfaff J, Herweh C, Pham M, Schieber S, Ringleb PA, et al. Combined proximal balloon occlusion and distal aspiration: a new approach to prevent distal embolization during neurothrombectomy. J Neurointerv Surg. 2017; 9:346–351.

32. Yoo AJ, Andersson T. Thrombectomy in acute ischemic stroke: challenges to procedural success. J Stroke. 2017; 19:121–130.

33. Baek SH, Kim S, Kang M, Choi JH, Kwon HJ, Kim DW. Effect of distal access catheter tip position on angiographic and clinical outcomes following thrombectomy using the combined stent-retriever and aspiration approach. PLoS One. 2021; 16:e0252641.

34. Pfaff JAR, Siekmann R, Shah YP, Ringleb PA, Ulfert C, Koller K, et al. Delivery assist catheters: a new device class and initial experience in mechanical thrombectomy in acute ischemic stroke patients. Clin Neuroradiol. 2019; 29:661–667.
Fig. 1.
Four elements constitute the catheters, from innermost to outmost layer: (1) liner, (2) reinforcement, (3) polymer jacket, and (4) hydrophilic coating. REACT aspiration catheters are constituted by a PTFE liner with low-frictional properties; hybrid (combined coil and braid) nitinol reinforcement that provides pushability, torqueability, flexibility, and resistance to kinking and ovalization; an outer jacket made of polymer blends with varying stiffnesses along the catheter shaft; and a hydrophilic coating on the distal segment that diminishes the friction between the device and vascular walls. PTFE, polytetrafluoroethylene, commonly known as Teflon.
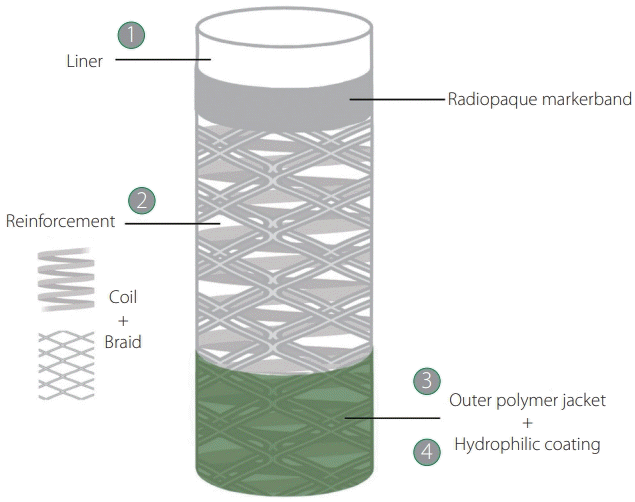
Table 1.
Clinical studies using REACT catheters
| Demographics and procedural data |
Reference |
||
|---|---|---|---|
| Raymond et al. [10] (2020) | Gross et al. [11] (2021) | Requena et al. [9] (2022) | |
| Number of patients | 47 | 150 | 102 |
| Median age | 67 | 71 | 78 |
| Sex, female | 16 (34) | 68 (45) | 51 (50) |
| Occlusion location | |||
| Tandem | 5 (11) | 22 (15) | 15 (15) |
| Anterior | 42 (89) | 130 (87) | 96 (94) |
| Posterior | 5 (11) | 20 (13) | 6 (6) |
| Technique | Aspiration/combined technique | Aspiration/combined technique | Combined technique |
| Device | |||
| REACT 68 | 47 (100) | NA | 23 (22) |
| REACT 71 | NA | 150 (100) | 79 (78) |
| Mean/median number of passes | 2 | 2.2 | 2 |
| Mean/median procedural time (min) | 22 | 25 | 40 |
| FPE (mTICI 2b–3) | NR | 76 (51) | 55 (54) |
| FPE (mTICI 2c–3) | NR | 39 (26) | 37 (36) |
| Successful recanalization (mTICI 2b-3) | 45 (96) | 142 (95) | 91 (89) |
| Complete recanalization (mTICI 2c–3) | NR | 59 (39) | 66 (65) |
| Complications | 2 (4) | 7 (5) | 4 (4) |
| 90-days mRS 0–2 | 24% | 34% | 36% |
Table 2.
Comparison of the build elements and key performance metrics among various commercially available aspiration catheters
| Parameters |
Device |
|||||
|---|---|---|---|---|---|---|
| REACT 68 | ACE 68 | Sofia Plus | REACT 71 | JET 7 | ||
| Build elements* | ||||||
| Liner | PTFE | PTFE | PTFE+polyolefin | PTFE+polyolefin | PTFE | |
| Reinforcement | NiTi | SS+NiTi | SS | NiTi | SS+NiTi | |
| Coil | Coil | Coil+braid | Coil+braid | Coil | ||
| Polymer jacket | PEBA, polyamide | Polyurethane, PEBA, polyamide | Polyurethane, PEBA, polyamide | Polyurethane, polyolefin, polyamide | Polyurethane, PEBA, polyamide | |
| Coating | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | |
| Aspiration [21,22] | ||||||
| Labeled ID (in) | 0.068 | 0.068 | 0.070 | 0.071 | 0.072 | |
| Effective ID (in) † | 0.081 | 0.079 | 0.083 | 0.089 | 0.083 | |
| Mean flow rate (mL/s) | NR | NR | 5.93 | 6.06 | 6.29 | |
| Mean tip force (mN) † | 373.65 | 338.10 | 392.21 | 455.43 | 396.75 | |
| Navigability [23] ‡ | ||||||
| SNAKE (mN) | 732.70 | 893.19 | 313.85 | 499.28 | 577.31 | |
| Microcatheter (mN) | 392.52 | 1111.29 | 285.89 | 299.08 | 388.59 | |
| SR anchoring (mN) | 264.34 | 543.02 | 195.20 | 147.77 | 257.94 | |
PTFE, polytetrafluoroethylene, commonly known as Teflon; NiTi, nitinol; SS, stainless steel; PEBA, polyether block amide, commonly known as Pebax; ID, inner diameter; NR, not reported; SNAKE, sofia non-wire advancement technike; SR, stent retriever; FDA, food and drug administration.
* Data retrieved from FDA 510(k) database. The exact compositions of the polymer blends and hydrophilic coatings were not disclosed.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download