Abstract
Background
Methotrexate is an antimetabolite drug that blocks dihydrofolate reductase and impairs cellular DNA synthesis. Administration of intravenous iodinated radiocontrast agents can cause life-threatening toxicity in patients receiving methotrexate.
Case
A 60-year-old female patient with rheumatoid arthritis underwent a craniotomy and clipping of a distal anterior cerebral artery aneurysm. The patient had been on low-dose oral methotrexate for the previous 5 years, which was discontinued two days before surgery. The patient received the first intravenous contrast agent injection (iohexol) during diagnostic cerebral angiography one day prior to surgery (50 ml) and the second contrast dose on the first postoperative day (60 ml). The patient developed severe methotrexate toxicity, leading to fatal multiorgan failure and death following repeated contrast imaging with intravenous iohexol.
Methotrexate (MTX) is an immunosuppressive agent indicated for malignant and chronic inflammatory states [1,2]. It is used as a disease-modifying agent in patients with rheumatoid arthritis to halt disease progression and provide symptomatic relief. Low-dose MTX (15–25 mg per week) is usually effective for rheumatoid arthritis. Toxic effects can be seen if the daily dose exceeds 500 mg/m2 of the body surface area, which manifests as mucocutaneous lesions and life-threatening multiorgan dysfunction [3]. We report a case of severe MTX toxicity leading to fatal multiorgan dysfunction in a patient on low-dose oral MTX who received two doses of an iodinated contrast agent.
The authors certify that written informed consent for publication was obtained from the patient or guardian.
A 60-year-old woman diagnosed with a distal anterior cerebral artery aneurysm was scheduled for a craniotomy and clipping of the aneurysm. The patient was known to have rheumatoid arthritis and to be taking 15 mg of oral MTX per week for the previous five years, the last dose of which was taken two days prior to admission. A diagnostic cerebral angiogram was performed on admission with 50 ml of intravenous iohexol (Omnipaque, 350 mg iodine/ml, GE Healthcare Pvt. Ltd, India) before surgery. Preoperative blood test results were unremarkable, and echocardiography revealed normal contractility, with an ejection fraction of 60%. The patient successfully underwent aneurysm clipping the day after admission, and the trachea was extubated following an uneventful intraoperative course. The patient was monitored in the neurocritical care unit in the immediate postoperative period and was treated with intravenous levetiracetam, mannitol, and paracetamol.
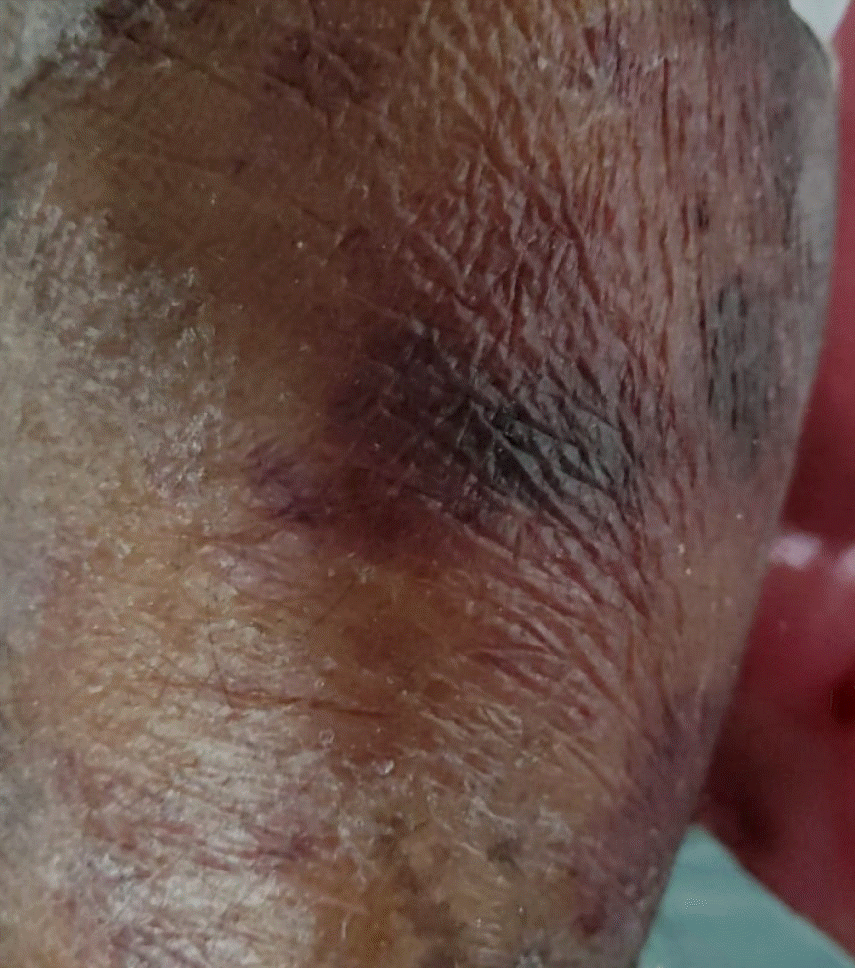
Gross abdominal distension was noted on the first postoperative day. Her blood test results were normal, except for a serum potassium level of 2 mmol/L. Emergent contrast-enhanced computed tomography (CECT) of the abdomen was performed with 60 ml of intravenous iohexol to rule out other possibilities. As the CECT of the abdomen was unremarkable, a diagnosis of postoperative paralytic ileus was considered, and intravenous potassium correction was initiated. The patient developed diffuse erythematous skin rashes 24 h after the CECT of the abdomen was conducted (Fig. 1). Suspecting an adverse drug event, antibiotics and anticonvulsants were temporarily withheld. On the third postoperative day, an increase in liver enzymes and doubling of creatinine levels were noted. The blood counts revealed severe pancytopenia with hemoglobin, leukocyte, and platelet counts of 6.6 g/dl, 3.4 × 109/L, and 42 × 109/L, respectively. MTX toxicity due to repeated contrast agent exposure was suspected, and the patient was hydrated with intravenous fluids to maintain a positive balance. As per the rheumatologist’s advice, leucovorin rescue was initiated at 75 mg/day in divided doses. However, in the subsequent days, the patient suffered a progressive decline in cell counts with worsening hepatic and renal parameters. On the fifth postoperative day, the patient required trachea intubation and lung ventilation owing to her deteriorated sensorium. An emergent plain computed tomography (CT) brain scan was performed, which did not reveal any apparent detectable pathology. The patient developed severe hypotension on the same day (postoperative day 5) and bedside echocardiography revealed global hypokinesia with mild pericardial effusion. Stabilization of the patient’s hemodynamics required supramaximal doses of intravenous noradrenaline and dobutamine. The patient developed severe bradycardia and cardiac arrest within a few hours, with no return of spontaneous circulation, despite cardiopulmonary resuscitation.
The clinical manifestations of severe MTX toxicity include pancytopenia due to bone marrow suppression, skin rashes, acute renal tubular failure due to crystallization of MTX in the renal tubules, intracranial bleeding, leukoencephalopathy, and cardiac failure, leading to multiorgan dysfunction and rapid progression to death. In patients receiving high-dose intravenous MTX, toxicity can be triggered by an intravenous iodinated contrast agent [4,5]. Non-steroidal anti-inflammatory drugs can induce similar adverse reactions in patients taking low-dose MTX [6]. However, fatal MTX toxicity following radiocontrast administration in patients receiving low-dose oral MTX has not been described previously. The differential diagnoses for the initial mucocutaneous lesions included toxic epidermal necrolysis, pemphigus vulgaris, and severe systemic lupus erythematosus. This patient had a long history of MTX ingestion, and the symptoms were temporally associated with contrast drug administration. The mucocutaneous lesions also preceded organ dysfunction. All of these findings suggested MTX toxicity and helped to rule out other possibilities. The patient received the first contrast injection 18 h prior to the surgery and the second dose 24 h after the surgery. The administration of mannitol (nephrotoxic agent) was the key precipitating event. Intravenous hydration, alkalinization of the urine, and leucovorin rescue are the standard treatment protocols for MTX toxicity. However, despite these measures, the patient could not be saved.
MTX-induced cerebral leukoencephalopathy can result in stroke-like symptoms or a sudden decrease in sensorium levels [7]. The sensorium of our patient deteriorated in the late stages despite a normal brain CT, which can be attributed to the above factors. Following oral ingestion, MTX undergoes rapid intracellular uptake in various tissues and is transformed to methotrexate polyglutamate, which inhibits the dihydrofolate reductase enzyme [8]. Even though the red blood cell concentration of methotrexate polyglutamate falls rapidly following oral cessation, there can be a slow and sustained release of the molecules from various tissue storage sites into the circulation, which can predispose a patient to toxicity by factors such as dehydration and renal dysfunction by delaying drug clearance. Glucarpidase is an MTX antidote that reduces extracellular MTX and facilitates its elimination [9]. Owing to its unavailability, this agent was not administered to the patient. To avoid dangerous drug interactions, it is recommended that the administration of contrast agents be delayed until the plasma MTX concentration falls below 0.05 mmol/L; therefore, waiting a minimum of 3–7 days between the last dose of MTX and the administration of a contrast agent is imperative [4]. However, as ruptured intracranial aneurysms require urgent treatment, as in this case, surgery cannot always be delayed and serum level estimations are not routinely available.
In conclusion, fatal multiorgan dysfunction can occur in patients receiving low-dose oral MTX who undergo contrast imaging; thus, extreme vigilance is necessary.
References
1. Czarnecka-Operacz M, Sadowska-Przytocka A. The possibilities and principles of methotrexate treatment of psoriasis - the updated knowledge. Postepy Dermatol Alergol. 2014; 31:392–400.

2. Weinblatt ME. Methotrexate: who would have predicted its importance in rheumatoid arthritis? Arthritis Res Ther. 2018; 20:103.

3. Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985; 312:818–22.

4. Schultz TE, Lynch AC. Intravenous radiographic contrast administered prior to high-dose methotrexate and subsequent toxicity requiring the use of glucarpidase. J Oncol Pharm Pract. 2019; 25:993–7.

5. Harned TM, Mascarenhas L. Severe methotrexate toxicity precipitated by intravenous radiographic contrast. J Pediatr Hematol Oncol. 2007; 29:496–9.

6. Jariwala P, Kumar V, Kothari K, Thakkar S, Umrigar DD. Acute methotrexate toxicity: a fatal condition in two cases of psoriasis. Case Rep Dermatol Med. 2014; 2014:946716.

7. Cruz-Carreras MT, Chaftari P, Shamsnia A, Guha-Thakurta N, Gonzalez C. Methotrexate-induced leukoencephalopathy presenting as stroke in the emergency department. Clin Case Rep. 2017; 5:1644–8.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download