INTRODUCTION
MATERIALS AND METHODS
Taurine Challenge Test after Treatment
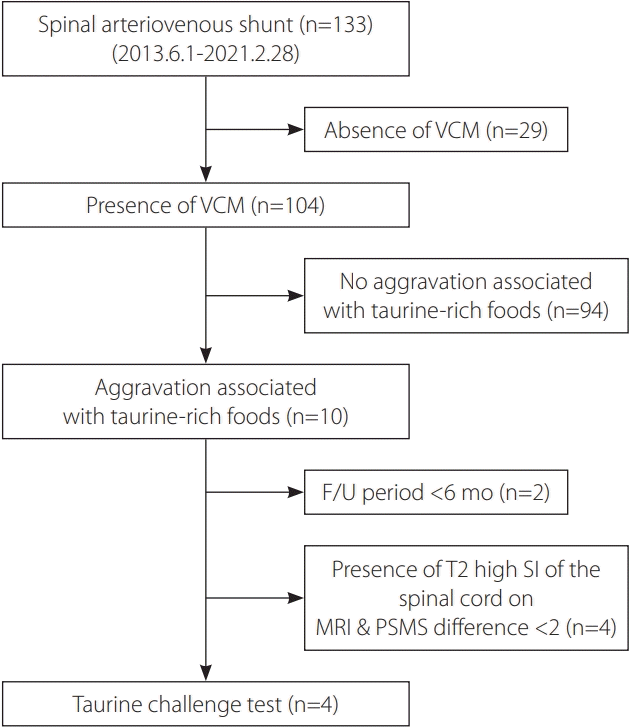 | Fig. 1.Candidate selection process diagram for the taurine challenge test. VCM, venous congestive myelopathy; F/U, follow-up; SI, signal intensity; MRI, magnetic resonance imaging; PSMS, pain-sensory-motor-sphincter score. |
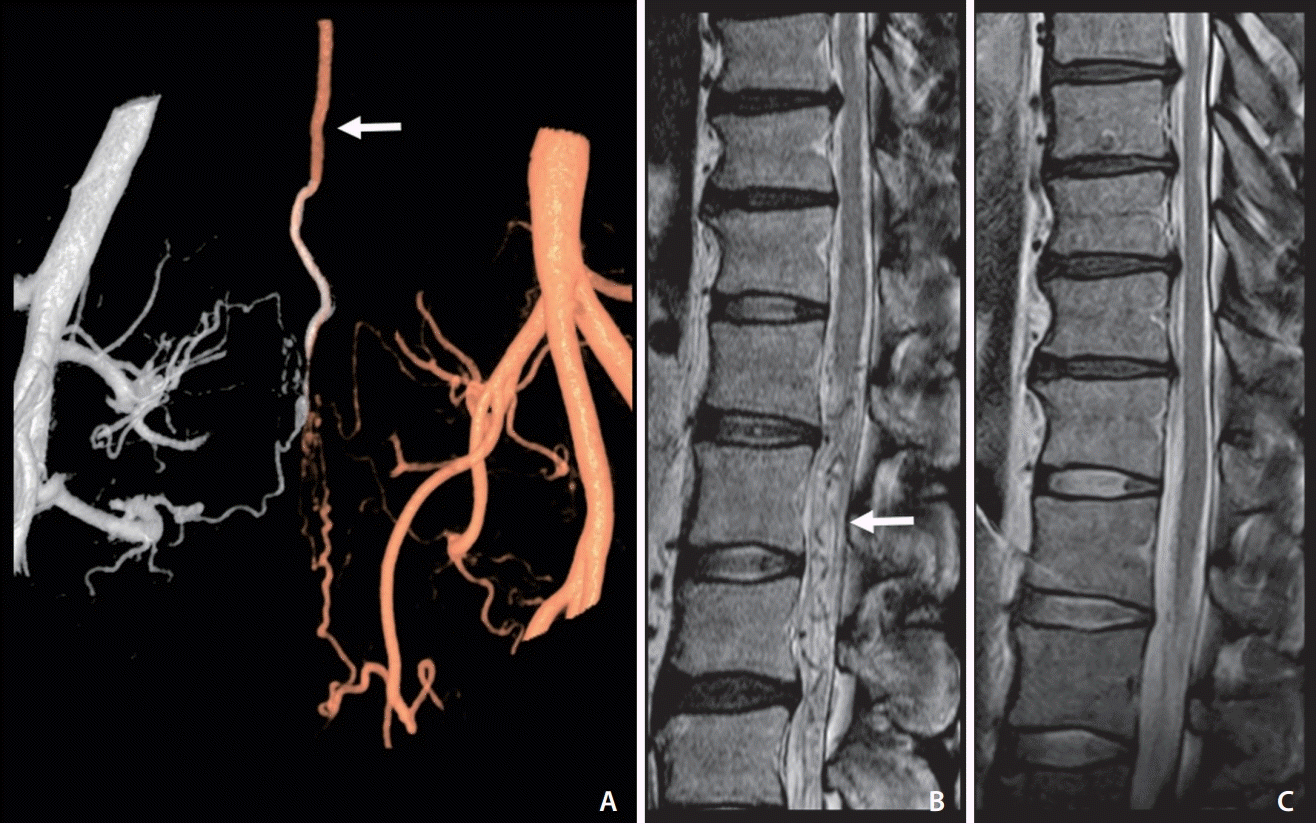 | Fig. 2.An adult patient with reversible aggravation myelopathy symptoms that developed after intake of taurine-rich foods and disappeared after treatment. (A) A fusion 3D angiogram of both internal iliac arteries shows a sacral dural arteriovenous shunt with retrograde regurgitation via the radicular vein (arrow). (B) Venous congestive myelopathy was noted in the spinal cord on a T2-weighted image in magnetic resonance imaging (MRI). Note the signal voids of the vessels around and below the swollen spinal cord (arrow). (C) Follow-up MRI after shunt treatment shows the disappearance of high signal intensities on the spinal cord and also revealed no symptom development after the intake of taurine-rich food. |
Table 1.
| Case | Age/Sex | Dx. | Lesion level | Steroid* | Taurine-rich foods | Pre-PSMS | Post-PSMS | Pre–Post PSMS† | Taurine challenge after Tx. | Free taurinerich food intake | F/U (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42/M | SDAVF | S2 | Yes | Webfoot octopus, squid, small octopus, Bacchus® 2,000 mg | 3 | 1 | 2 | Done at 24 months | Yes | 24 |
| 2 | 71/M | SDAVF | T10 | NA | Webfoot octopus, squid, small octopus, crab, scallops | 4 | 2 | 2 | Done at 26 months | Yes | 26 |
| 3 | 63/F | SEDAVF | L2 | NA | Bacchus® 1,000 mg | 4 | 1 | 3 | Done at 48 months | Yes | 48 |
| 4 | 45/M | SDAVF | L1 | No | Bacchus® 2,000 mg | 5 | 0 | 5 | Done at 76 months | Yes | 76 |
Bacchus® (Dong-A Pharmaceutical, Seoul, Korea).
VCM, venous congestive myelopathy; Dx., diagnosis; PSMS, pain-sensory-motor-sphincter score; Tx., treatment; F/U, follow-up; M, male; F, female; SDAVF, spinal dural arteriovenous fistula; SEDAVF, spinal epidural arteriovenous fistula; NA, not available.
RESULTS
Taurine Challenge Test
DISCUSSION
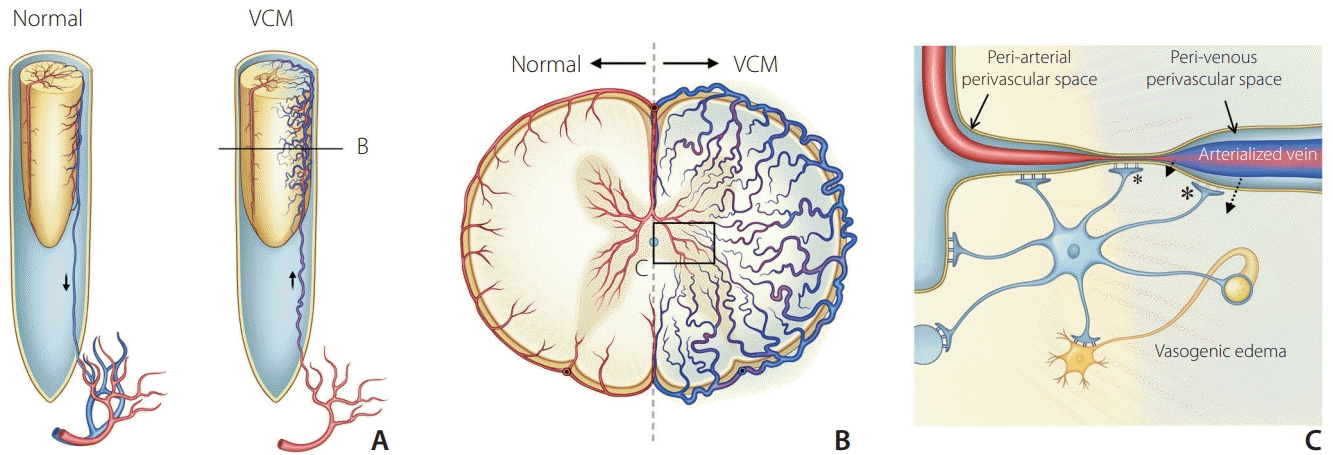 | Fig. 3.Schematic diagram of possible mechanism related to the vasogenic edema in venous congestive myelopathy (VCM). (A) Arterialized venous engorgement around the spinal cord and cord swelling in the spinal dural arteriovenous shunt. (B) Cross-section of the swollen spinal cord showed venous engorgement of arterialized veins with decreased arterial perfusion in the VCM. (C) Magnified view of the vasogenic edema shows increased hydrostatic pressure caused by venous hypertension that opens Aquaporin-4 water channels in the foot processes of astrocytes and induces perivascular water movement (dotted arrows). Damage of blood-spinal cord barrier at the venous perivascular space (large asterisk) and capillary wall (small asterisk) finally increases interstitial fluid in the extracellular space leading to vasogenic edema. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download