1. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016; 17:836–44.

2. Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010; 139:976–81.

3. Maxwell C, Nicoara A. New developments in the treatment of acute pain after thoracic surgery. Curr Opin Anaesthesiol. 2014; 27:6–11.

4. Neustein SM, McCormick PJ. Postoperative analgesia after minimally invasive thoracoscopy: what should we do? Can J Anaesth. 2011; 58:423–5, 425-7.

5. Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008; 107:1026–40.

6. Kaiser AM, Zollinger A, De Lorenzi D, Largiadèr F, Weder W. Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg. 1998; 66:367–72.

7. Helms O, Mariano J, Hentz JG, Santelmo N, Falcoz PE, Massard G, et al. Intra-operative paravertebral block for postoperative analgesia in thoracotomy patients: a randomized, double-blind, placebo-controlled study. Eur J Cardiothorac Surg. 2011; 40:902–6.

8. Rawal N. Epidural technique for postoperative pain: gold standard no more? Reg Anesth Pain Med. 2012; 37:310–7.
9. Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014; 18:626–35.

10. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006; 96:418–26.

11. Daly DJ, Myles PS. Update on the role of paravertebral blocks for thoracic surgery: are they worth it? Curr Opin Anaesthesiol. 2009; 22:38–43.

12. Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011; 115:181–8.

13. Batchelor TJ, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019; 55:91–115.

14. Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008; 107:1026–40.

15. Chin KJ, Adhikary SD, Forero M. Erector spinae plane (ESP) block: a new paradigm in regional anesthesia and analgesia. Curr Anesthesiol Rep. 2019; 9:271–80.

16. Park MH, Kim JA, Ahn HJ, Yang MK, Son HJ, Seong BG. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. 2018; 73:1260–4.

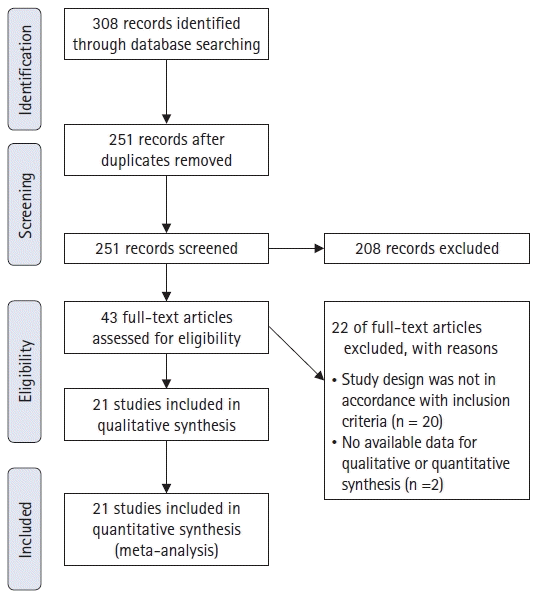
17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1.

18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135.

19. Shim SR, Kim SJ, Lee J, Rucker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019; 41:e2019013.

20. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. Boca Raton: Chapman & Hall/CRC Press;2021.
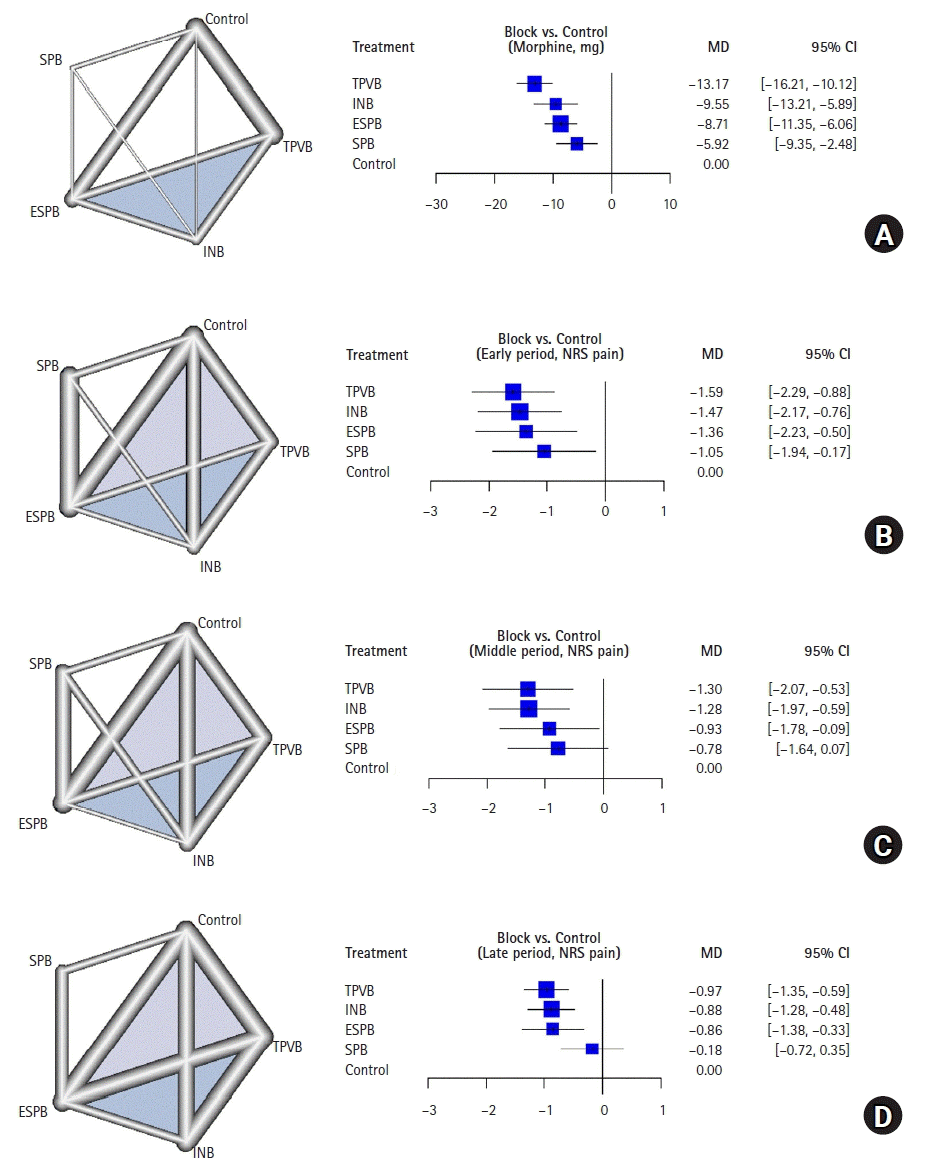
21. Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014; 33:3639–54.

22. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015; 15:58.

23. Papakonstantinou T, Nikolakopoulou A, Higgins JP, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020; 16:e1080.

24. Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020; 17:e1003082.

25. Liu L, Ni XX, Zhang LW, Zhao K, Xie H, Zhu J. Effects of ultrasound-guided erector spinae plane block on postoperative analgesia and plasma cytokine levels after uniportal VATS: a prospective randomized controlled trial. J Anesth. 2021; 35:3–9.

26. Hu L, Xu X, Tian H, He J. Effect of single-injection thoracic paravertebral block via the intrathoracic approach for analgesia after single-port video-assisted thoracoscopic lung wedge resection: a randomized controlled trial. Pain Ther. 2021; 10:433–42.

27. Zhao H, Xin L, Feng Y. The effect of preoperative erector spinae plane vs. paravertebral blocks on patient-controlled oxycodone consumption after video-assisted thoracic surgery: a prospective randomized, blinded, non-inferiority study. J Clin Anesth. 2020; 62:109737.

28. Yao Y, Fu S, Dai S, Yun J, Zeng M, Li H, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth. 2020; 63:109783.

29. Viti A, Bertoglio P, Zamperini M, Tubaro A, Menestrina N, Bonadiman S, et al. Serratus plane block for video-assisted thoracoscopic surgery major lung resection: a randomized controlled trial. Interact Cardiovasc Thorac Surg. 2020; 30:366–72.

30. Turhan Ö, Sivrikoz N, Sungur Z, Duman S, Özkan B, Şentürk M. Thoracic paravertebral block achieves better pain control than erector spinae plane block and intercostal nerve block in thoracoscopic surgery: a randomized study. J Cardiothorac Vasc Anesth. 2021; 35:2920–7.

31. Lee J, Lee DH, Kim S. Serratus anterior plane block versus intercostal nerve block for postoperative analgesic effect after video-assisted thoracoscopic lobectomy: a randomized prospective study. Medicine (Baltimore). 2020; 99:e22102.
32. Kim S, Bae CM, Do YW, Moon S, Baek SI, Lee DH. Serratus anterior plane block and intercostal nerve block after thoracoscopic surgery. Thorac Cardiovasc Surg. 2021; 69:564–9.

33. Finnerty DT, McMahon A, McNamara JR, Hartigan SD, Griffin M, Buggy DJ. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth. 2020; 125:802–10.

34. Çiftçi B, Ekinci M, Çelik EC, Tukac İC, Gölboyu BE, Günlüoğlu MZ, et al. Ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia management following video-assisted thoracic surgery: a prospective, randomized, controlled study. Anestezi Dergisi. 2020; 28:170–8.

35. Ciftci B, Ekinci M, Celik EC, Tukac IC, Bayrak Y, Atalay YO. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: a prospective randomized study. J Cardiothorac Vasc Anesth. 2020; 34:444–9.

36. Chu H, Dong H, Wang Y, Niu Z. Effects of ultrasound-guided paravertebral block on MMP-9 and postoperative pain in patients undergoing VATS lobectomy: a randomized, controlled clinical trial. BMC Anesthesiol. 2020; 20:59.

37. Cheng XQ, Zhang MY, Fang Q, Shi DW, Huang XC, Liu XS, et al. Opioid-sparing effect of modified intercostal nerve block during single-port thoracoscopic lobectomy: Retraction: A randomised controlled trial. Eur J Anaesthesiol 2021. Advance Access published on Sep 9, 2021. doi: 10.1097/EJA.0000000000001394. Retraction in: Eur J Anaesthesiol 2021; 38: 677.
38. Chen N, Qiao Q, Chen R, Xu Q, Zhang Y, Tian Y. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: a randomized, double-blinded, clinical trial. J Clin Anesth. 2020; 59:106–11.

39. Gaballah KM, Soltan WA, Bahgat NM. Ultrasound-guided serratus Plane Block versus Erector spinae block for postoperative analgesia after video-assisted thoracoscopy: a pilot randomized controlled trial. J Cardiothorac Vasc Anesth. 2019; 33:1946–53.

40. Wu C, Ma W, Cen Q, Cai Q, Wang J, Cao Y. A comparison of the incidence of supraventricular arrhythmias between thoracic paravertebral and intercostal nerve blocks in patients undergoing thoracoscopic surgery: a randomised trial. Eur J Anaesthesiol. 2018; 35:792–8.
41. Ökmen K, Metin Ökmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med. 2018; 37:349–53.

42. Kim DH, Oh YJ, Lee JG, Ha D, Chang YJ, Kwak HJ. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. 2018; 126:1353–61.
43. Ahmed Z, Samad K, Ullah H. Role of intercostal nerve block in reducing postoperative pain following video-assisted thoracoscopy: a randomized controlled trial. Saudi J Anaesth. 2017; 11:54–7.

44. Kaya FN, Turker G, Basagan-Mogol E, Goren S, Bayram S, Gebitekin C. Preoperative multiple-injection thoracic paravertebral blocks reduce postoperative pain and analgesic requirements after video-assisted thoracic surgery. J Cardiothorac Vasc Anesth. 2006; 20:639–43.

45. Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005; 95:816–21.
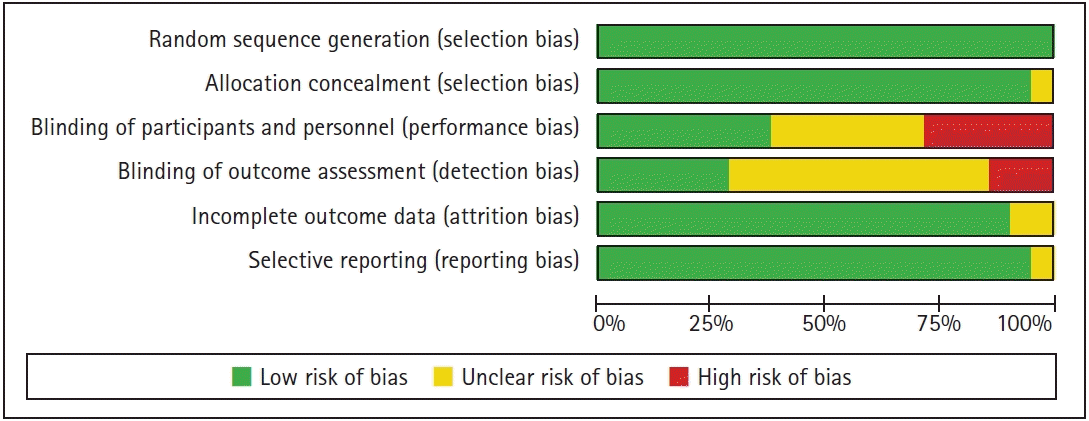
46. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.

47. Hussain N, Brull R, Noble J, Weaver T, Essandoh M, McCartney CJ, et al. Statistically significant but clinically unimportant: a systematic review and meta-analysis of the analgesic benefits of erector spinae plane block following breast cancer surgery. Reg Anesth Pain Med. 2021; 46:3–12.

48. Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017; 118:424–9.

49. Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. J Clin Anesth. 2020; 66:109900.

50. Fanelli A, Torrano V, Cozowicz C, Mariano ER, Balzani E. The opioid sparing effect of erector spinae plane block for various surgeries: a meta-analysis of randomized-controlled trials. Minerva Anestesiol. 2021; 87:903–14.

51. Hong B, Bang S, Chung W, Yoo S, Chung J, Kim S. Multimodal analgesia with multiple intermittent doses of erector spinae plane block through a catheter after total mastectomy: a retrospective observational study. Korean J Pain. 2019; 32:206–14.

52. Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021; 68:387–408.

53. Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med. 2020; 45:10–5.

54. Hong B, Lim C, Kang H, Eom H, Kim Y, Cho HJ, et al. Thoracic paravertebral block with adjuvant dexmedetomidine in video-assisted thoracoscopic surgery: a randomized, double-blind study. J Clin Med. 2019; 8:352.

55. Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019; 7:668.

56. Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016; 2:CD009121.

57. Wong HY, Pilling R, Young BW, Owolabi AA, Onwochei DN, Desai N. Comparison of local and regional anesthesia modalities in breast surgery: a systematic review and network meta-analysis. J Clin Anesth. 2021; 72:110274.

58. Chin KJ, Pawa A, Forero M, Adhikary S. Ultrasound-guided fascial plane blocks of the thorax: pectoral I and II, serratus anterior plane, and erector spinae plane blocks. Adv Anesth. 2019; 37:187–205.
59. Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth. 2017; 31:152–8.

60. Ramamurthy S, Hickey R, Maytorena A, Hoffman J, Kalantri A. Long thoracic nerve block. Anesth Analg. 1990; 71:197–9.

61. Kwon WK, Choi JW, Kang JE, Kang WS, Lim JA, Woo NS, et al. Long thoracic nerve block in video-assisted thoracoscopic wedge resection for pneumothorax. Anaesth Intensive Care. 2012; 40:773–9.

62. Bremner-Smith AT, Unwin AJ, Williams WW. Sensory pathways in the spinal accessory nerve. J Bone Joint Surg Br. 1999; 81:226–8.

63. Porzionato A, Macchi V, Stecco C, Loukas M, Tubbs RS, De Caro R. Surgical anatomy of the pectoral nerves and the pectoral musculature. Clin Anat. 2012; 25:559–75.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download