Abstract
Background
The thoracic retrolaminar block (TRLB) is a relatively new regional analgesia technique that can be used as an alternative to the thoracic paravertebral block. This study aimed to evaluate the postoperative analgesia effects of ultrasound-guided TRLB in children undergoing open cardiac surgery via median sternotomy incision.
Methods
Sixty-six patients aged 2–8 years were recruited. In the TRLB group, 0.25% bupivacaine 0.4 ml/kg was injected into the retrolaminar space on both sides at the level of the T4 lamina. Patients in the control group were injected with 0.9% saline. The primary outcome measure was fentanyl consumption in the first 24 h post-extubation. The secondary outcome measures were the total intraoperative fentanyl consumption, postoperative modified objective pain score (MOPS), and time to extubation.
Results
The total intraoperative fentanyl requirements and fentanyl consumption in the first 24 h post-extubation were significantly lower (P < 0.001) in the TRLB group (9.3 ± 1.2; 6.9 ± 2.1 μg/kg, respectively) than in the control group (12.5 ± 1.4; 16.6 ± 2.8, respectively). The median (Q1, Q3) time to extubation was significantly shorter (P < 0.001) in the TRLB group (2 [1, 3] h) than in the control group (6 [4.5, 6] h). The MOPS was significantly lower (P < 0.05) in the TRLB group than in the control group at 0, 2, 4, 8, 12 and 16 h post-extubation.
Cardiac surgery via a median sternotomy incision causes moderate to severe postoperative pain that can be prevented using an appropriate multimodal analgesic regimen [1]. Regional analgesia is an essential component of postoperative multimodal analgesia for all surgical patients, including children undergoing cardiac surgery. The use of ultrasound guidance in anesthesia increases the safety and efficacy of various regional anesthesia techniques [2]. Currently, the use of neuraxial analgesia, including epidural, caudal, and spinal analgesia, has been gradually replaced by safer ultrasound-guided fascial plane blocks, especially in cardiac surgery, to avoid the potential risk of epidural hematoma in fully anticoagulated patients [3,4].
Ultrasound-guided thoracic retrolaminar block (TRLB) is a relatively new regional analgesic block that can be used as an alternative to the thoracic paravertebral block as a component of multimodal analgesia to control postoperative pain [5]. In adults, the analgesic efficacy of TRLB has been reported for rib fractures [6], breast surgery [7,8], and video-assisted thoracoscopic surgery [9]. Injecting a local anesthetic in the retrolaminar space blocks the ventral and dorsal rami of the thoracic spinal nerves and spreads laterally in the fascial plane to block the lateral cutaneous branch of the intercostal nerve and the small branches arising from it [5]. The only pediatric randomized study on the use of TRLB for postoperative analgesia was conducted in patients undergoing inguinal hernia repair [10]. To the best of our knowledge, this is the first clinical trial to evaluate the analgesic efficacy of TRLB after open cardiac surgery via median sternotomy.
This prospective randomized controlled study was designed to demonstrate the postoperative analgesic effects of single-shot bilateral TRLB in terms of 24 h postoperative fentanyl consumption and pain scores. We hypothesized that the bilateral TRLB would decrease the postoperative fentanyl consumption and pain scores. The primary outcome measured was 24 h post-extubation fentanyl consumption, and the secondary outcomes were postoperative pain scores, time to first rescue analgesia, time to extubation, and the incidence of TRLB-related complications.
This was a single-center, prospective, randomized controlled, double-blind, superiority study with two parallel arms. The study protocol was approved by the Institutional Review Board of Mansoura Faculty of Medicine, Mansoura, Egypt (IRB code: R 20.11.1073) on December 3, 2020, and registered in the African clinical trial registry (PACTR202012621958228) before patient recruitment. Written informed consent to perform the retrolaminar block and publish this study was obtained from the patients’ legal guardians before surgery. This study was conducted in accordance with the ethical principles of the Helsinki Declaration-2013 and followed good clinical practice guidelines.
This study was conducted at the cardiac division of Mansoura University Children's Hospital between December 2020 and September 2021. A total of 66 patients aged 2–8 years (both male and female) with American Society of Anesthesiologists physical status classification I and II who were scheduled to undergo cardiac surgery via midline sternotomy by cardiopulmonary bypass (CPB) for the repair of simple congenital heart diseases were recruited. The exclusion criteria were as follows: history of multiple cardiac surgeries, history of emergency surgery, intubated patients, patients receiving inotropic support, and those with pulmonary hypertension, bleeding disorders, or an allergy to amide local anesthetics.
The patients were randomized to either the TRLB or control group using random computer-generated numbers with an allocation ratio of 1 : 1. The patient group assignment was kept in a sealed opaque envelope that was delivered to the operating room on the day of surgery and opened just before the induction of anesthesia. The anesthesiologist who prepared the local anesthetic and placebo was not involved in the study. The anesthesia residents who collected the data and the nursing staff who provided postoperative care were all blinded to patient group allocation.
Patients were premedicated with 0.5 mg/kg oral midazolam 30 min before separation from their parents. Patients were monitored before induction of anesthesia using 5-lead electrocardiography, pulse oximetry, and a non-invasive blood pressure cuff. Induction of anesthesia was performed either through inhaled sevoflurane or intravenous propofol 1.5 mg/kg depending on the presence or absence of an intravenous line and the patient’s age. Rocuronium 0.9 mg/kg and fentanyl 2 μg/kg were administered before tracheal intubation. A capnography was then connected to the endotracheal tube to monitor end-tidal CO2. A 3 Fr Leadercath (Vygon, France) was inserted into the femoral artery for invasive arterial pressure monitoring, a 5–5.5 Fr central venous catheter was inserted into the right internal jugular vein to monitor the central venous pressure, and a temperature probe was inserted into the nasopharynx to monitor the patient’s temperature.
Anesthesia was maintained with sevoflurane (1–2%) in a mixture of air-oxygen at a ratio of 1 : 1, fentanyl 1 μg/kg/h, and rocuronium 0.5 mg/kg/h. Additional 1 μg/kg boluses of fentanyl were administered before skin incision, before the sternotomy, and when the mean arterial blood pressure and/or heart rate increased by 20% above baseline.
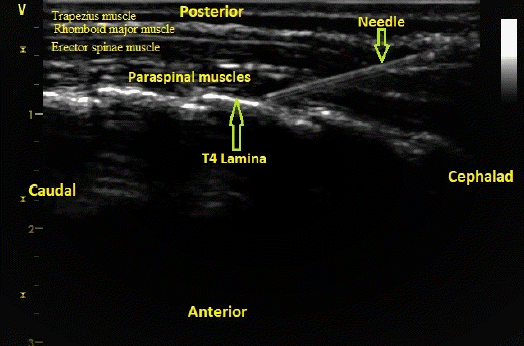
After anesthesia induction, the patient was placed in a prone position with a pillow under the chest to perform a retrolaminar block on both sides. The spinous process of the fourth thoracic vertebra was identified and marked. Ultrasound-guided TRLB was performed under complete sterilization using Sterillium® sterile drapes, and the ultrasound probe was placed in a sterile sheath. A GE Vivid S5 high-frequency ultrasound linear transducer (General Electric Ving Med Systems, Horten, Norway) was placed in a parasagittal position just lateral to the spinous processes of the thoracic vertebra to identify the trapezius, rhomboid major, erector spinae, and paraspinal muscles and the vertebral lamina (Fig. 1). A 50-mm 22 gauge sonographic needle (Stimuplex®, Braun Medical, USA) was inserted using the in-plane technique in the cephalad-to-caudal direction and advanced until the lamina of the fourth thoracic vertebra was touched, after which 0.25% bupivacaine 0.4 ml/kg was injected on each side (Fig. 1). In the control group, 0.9% normal saline 0.4 ml/kg was injected instead of 0.25% bupivacaine.
All surgeries were performed via midline sternotomy incision (Fig. 2A). Before initiation of CPB, each patient received 3–4 mg/kg heparin through the central venous catheter to increase the activated clotting time above 480 s. The presence of mild hypothermia was considered acceptable. After the cardiac defect was repaired, the patient was rewarmed, separated from CPB, and protamine was administered to antagonize the heparin. After the surgery was completed and the drainage tubes were placed (Fig. 2B), the patient was transferred to the intensive care unit (ICU).
The patients were monitored in the ICU using the same parameters as those used during surgery. Extubation was performed once the patient fulfilled the extubation criteria (adequate level of consciousness, normothermia, hemodynamic stability, minimal inotropic support, absence of significant bleeding, adequate spontaneous respiration, and acceptable arterial blood gas levels). Postoperative pain was assessed for the first 24 h after extubation using a 10-point modified objective pain score (MOPS) [11]. All patients received a standard protocol of multimodal analgesia in the form of paracetamol 15 mg/kg every 8 h and intravenous ibuprofen 10 mg/kg every 6 h. Fentanyl 1 μg/kg was administered as a rescue analgesic when the MOPS was > 3.
The primary outcome measure was fentanyl consumption in the first 24 h post-extubation. The secondary outcome measures were total intraoperative fentanyl consumption, postoperative pain scores at rest, time to extubation measured after arrival in the ICU, time to the first rescue analgesia measured after extubation, ICU length of stay, and the incidence of TRLB-related complications (e.g., hypotension, pneumothorax, vascular or neurological injury, or local anesthetic toxicity). The incidence of other complications (vomiting and pruritus) were also reported. Postoperative pain (according to the MOPS) was assessed at rest at 0, 2, 4, 8, 12, 16, and 24 h post-extubation.
The sample size was calculated using PASS version 15.0.5 software for Windows (PASS, LLC, USA), and a superiority test was conducted to determine the difference between two means. This study was designed as a superiority trial. Since no similar study has been conducted previously, we calculated the sample size using the results of our pilot study, which included six patients in each group (these patients are not included in this study). The mean ± SD fentanyl consumption in the first 24 h post-extubation in our pilot study was 7.5 ± 2.4 μg/kg in the TRLB group and 13 ± 4.3 μg/kg in the control group. Group sample sizes of 25 patients achieved 80% power to detect superiority using a one-sided two-sample t-test. The margin of superiority was 3 μg/kg. The true difference between the means was assumed to be 5.5 μg/kg. The significance level (α) of the test was 0.05. The data were drawn from populations with standard deviations of 2.4 and 4.3. The final sample size was increased to 32 patients per group to compensate for an estimated dropout rate of 20%.
Statistical analysis was performed using IBM SPSS (version 21.0; IBM Corp., USA) for Windows. The Shapiro-Wilk test was used to determine the normality of the data distribution. Normally distributed quantitative data are represented as the mean ± SD and were analyzed using the independent t-test. Non-parametric variables are reported as median (interquartile range [Q1, Q3]) and were analyzed using the Mann-Whitney U test. Categorical variables are expressed as numbers and were analyzed using the chi-square test. Statistical analyses were performed by comparing the TRLB and control groups. P < 0.05 was used to indicate statistical significance with a 95% CI.
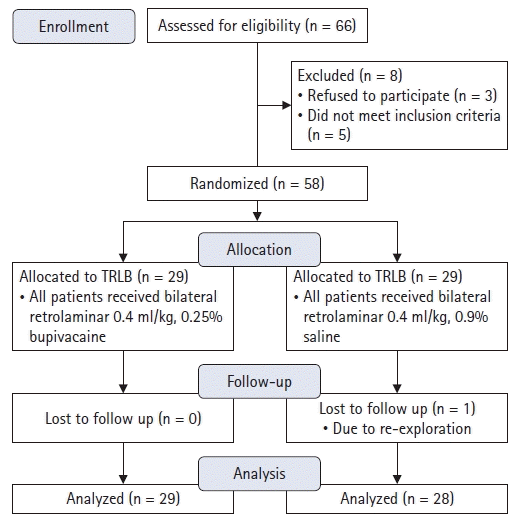
Sixty-six patients were recruited, of whom eight were excluded either due to refusal by their legal guardians (n = 3) or failing to meet the inclusion criteria (n = 5) (Fig. 3). One patient in the control group was lost to follow-up owing to re-exploration after developing postoperative surgical bleeding. Fifty-seven patients completed the final analysis, 29 in the TRLB group and 28 in the control group (Fig. 3).
There were no significant differences between the TRLB and control groups in terms of the patient and surgical characteristics (Table 1).
The analgesic profiles of the two study groups are compared in Table 2. The mean ± SD total intraoperative fentanyl consumption and fentanyl consumption in the first 24 h post-extubation were significantly lower (P < 0.001) in the TRLB group (9.3 ± 1.2 and 6.9 ± 2.1 μg/kg, respectively) than in the control group (12.5 ± 1.4 and 16.6 ± 2.8 μg/kg, respectively). The median (Q1, Q3) time to first rescue analgesia was significantly longer (P < 0.001) in the TRLB group (7 [5, 8] h) than in the control group (2 [1, 2] h). The median (Q1, Q3) time to extubation and the mean ± SD ICU length of stay were significantly shorter (P < 0.001) in the TRLB group (2 [1, 3] h and 23.8 ± 3.2 h, respectively) than in the control group (6 [4.5, 6] h and 30.3 ± 3.2 h, respectively) (Table 2). There were no TRLB-related complications reported (hypotension, pneumothorax, vascular or neurological injury, or local anesthetic toxicity) (Table 2). There was also no significant difference in the incidence of vomiting or pruritus between the control and TRLB groups.
Fig. 4 shows a comparison of the MOPS between the TRLB and control groups. The MOPS was significantly lower (P < 0.05) in the TRLB group than in the control group at 0, 2, 4, 8, 12 and 16 h post-extubation. At 24 h post-extubation, however, the MOPS was similar between the groups.
In this randomized controlled superiority study, 66 patients were examined for eligibility, nine of whom were excluded or lost to follow-up, leaving a total of 57 patients in the final analysis (n = 29 in the TRLB group and n = 28 in the control group). The main results of our study demonstrated that bilateral ultrasound-guided TRLB is associated with decreased perioperative fentanyl consumption, post-extubation pain scores, time to first analgesia request, time to extubation, and ICU length of stay.
Pediatric open cardiac surgery causes moderate to severe postoperative pain that arises mainly from the median sternotomy incision and, to a lesser extent, the drainage tube sites [12]. An appropriate multimodal analgesic regimen, including the use of a regional anesthetic technique, is usually used to control pain after cardiac surgery. Currently, most anesthetists prefer to use ultrasound-guided muscle plane blocks instead of spinal, caudal, epidural, or paravertebral analgesia due to the associated risk of epidural hematoma after full heparinization [3,4]. Bilateral ultrasound-guided erector spinae plane blocks [13-15] and transversus thoracis muscle plane blocks [16,17] are associated with effective postoperative analgesia after pediatric cardiac surgery. The present study is the first to use bilateral TRLB for analgesia after cardiac surgery via midline sternotomy incision.
The retrolaminar block is a simple and easy to perform fascial plane block that involves the deposition of local anesthetics between the posterior surface of the thoracic vertebral lamina and overlying paraspinal muscles [18]. In this study, we injected a relatively large volume of 0.25% bupivacaine (0.4 ml/kg) because the distribution of local anesthetics and the analgesic efficacy of TRLB are volume-dependent [19]. The injectate increases the pressure in the non-compliant retrolaminar space, allowing for the ventral spread of local anesthetics into the paravertebral and epidural spaces to block the dorsal and ventral rami of the spinal nerves. The injectate also spreads laterally in the fascial plane along the ventral surface of the erector spinae muscle to block the cutaneous and small branches of the intercostal nerves, producing analgesia in the hemithorax [5]. Currently, the exact mechanism of TRLB analgesia is not completely understood. We postulated that the principal mechanism is the spread of the injected local anesthetic to the paravertebral and epidural spaces, producing analgesia similar to or somewhat inferior to that of paravertebral blocks.
Several studies have demonstrated the analgesic efficacy of TRLB. Nobukuni et al. [20] compared the postoperative efficacy of TRLB and thoracic epidural analgesia after video-assisted thoracoscopic surgery and found that TRLB was as effective as epidural analgesia in controlling postoperative pain in terms of pain scores and opioid consumption. Sotome et al. [21] found that the postoperative analgesic effects of TRLB were equivalent to those of erector spinae plane blocks after breast surgery. Zhao et al. [22] found that the analgesic effects of TRLB were superior to those of erector spinae blocks in patients with multiple rib fractures. Wang et al. [9] compared ultrasound-guided TRLBs and paravertebral blocks for postoperative analgesia in patients undergoing video-assisted thoracoscopic surgery and found that the paravertebral block resulted in better analgesia than the TRLB. In contrast, Hwang et al. [23] conducted a randomized placebo study that aimed to assess the analgesic efficacy of a single injection of ultrasound-guided TRLB after breast surgery and reported that TRLB did not reduce postoperative analgesic consumption. We postulate that the lack of efficacy of TRLB in reducing opioid consumption after radical mastectomy could be attributed to the complexity of the surgery, which includes axillary lymphadenectomy.
In our study, TRLB was associated with a reduced time to extubation and ICU length of stay. This is consistent with other chest wall fascial plane blocks, including the bilateral erector spinae plane block [13-15] and the transversus thoracis muscle plane block [16,17]. These findings could be explained by the reduction in perioperative opioid consumption. Decreasing the time to extubation and ICU length of stay allows for fast-track cardiac surgery, decreases costs, and saves resources.
In this study, TRLB complications (hypotension, pneumothorax, vascular or neurological injury, and local anesthetic toxicity) were not reported. Ultrasound-guided TRLB is simple, easy to perform, and theoretically safer than thoracic epidural analgesia and paravertebral blocks since the block needle is inserted towards the vertebral lamina and thus away from any important vessels, the pleura, and the dura. Additionally, sonographic visualization of the block needle is better in children than in adults.
Our study had several limitations. First, the sample size was small in this single-center study, and we could thus not determine the actual incidence of TRLB complications. Future multicenter studies with larger sample sizes are recommended. Second, we did not assess the dermatomal spread of the TRLB because we performed TRLBs in children after anesthesia induction. Further studies in conscious adults are necessary. Third, the smallest effective volume of local anesthetics is unknown; we used a relatively large volume (0.4 ml/kg) of 0.25% bupivacaine to ensure the efficacy of the block. Fourth, a single injection of a local anesthetic was used in this study, which resulted in a limited duration of analgesia. Therefore, continuous infusions of local anesthetics is recommended in future studies to obtain more prolonged analgesia. Finally, we did not measure the serum concentration of bupivacaine because of the unavailability of the above technology in our institutional hospital.
Based on the findings of this study, we conclude that ultrasound-guided bilateral TRLB performed at the level of the fourth thoracic vertebra is effective in providing postoperative analgesia in terms of opioid consumption and postoperative pain scores in children undergoing open cardiac surgery via median sternotomy incision. Additionally, TRLB is associated with early tracheal extubation and a short ICU length of stay.
Notes
Author Contributions
Ibrahim Abdelbaser (Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft; Writing – review & editing)
Nabil A. Mageed (Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing – review & editing)
Sherif I. Elfayoumy (Software)
Mohamed Magdy (Visualization)
Mohamed M. Elmorsy (Resources)
Mahmoud M. ALseoudy (Data curation)
References
1. Barr LF, Boss MJ, Mazzeffi MA, Taylor BS, Salenger R. Postoperative multimodal analgesia in cardiac surgery. Crit Care Clin. 2020; 36:631–51.

2. Jeon YH. Easier and safer regional anesthesia and peripheral nerve block under ultrasound guidance. Korean J Pain. 2016; 29:1–2.

3. Bösenberg A. Neuraxial blockade and cardiac surgery in children. Paediatr Anaesth. 2003; 13:559–60.

4. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018; 43:263–309. Erratum in: Reg Anesth Pain Med 2018; 43: 566.

5. Onishi E, Toda N, Kameyama Y, Yamauchi M. Comparison of clinical efficacy and anatomical investigation between retrolaminar block and erector spinae plane block. Biomed Res Int. 2019; 2019:2578396.

6. Voscopoulos C, Palaniappan D, Zeballos J, Ko H, Janfaza D, Vlassakov K. The ultrasound-guided retrolaminar block. Can J Anaesth. 2013; 60:888–95.

7. Jüttner T, Werdehausen R, Hermanns H, Monaca E, Danzeisen O, Pannen BH, et al. The paravertebral lamina technique: a new regional anesthesia approach for breast surgery. J Clin Anesth. 2011; 23:443–50.

8. Murouchi T, Yamakage M. Retrolaminar block: analgesic efficacy and safety evaluation. J Anesth. 2016; 30:1003–7.

9. Wang Q, Wei S, Li S, Yu J, Zhang G, Ni C, et al. Comparison of the analgesic effect of ultrasound-guided paravertebral block and ultrasound-guided retrolaminar block in Uniportal video-assisted thoracoscopic surgery: a prospective, randomized study. BMC Cancer. 2021; 21:1229.

10. Alseoudy MM, Abdelbaser I. Ultrasound-guided retrolaminar block versus ilioinguinal nerve block for postoperative analgesia in children undergoing inguinal herniotomy: a randomized controlled trial. J Clin Anesth. 2021; 74:110421.

11. Wilson GA, Doyle E. Validation of three paediatric pain scores for use by parents. Anaesthesia. 1996; 51:1005–7.

12. Lahtinen P, Kokki H, Hynynen M. Pain after cardiac surgery: a prospective cohort study of 1-year incidence and intensity. Anesthesiology. 2006; 105:794–800.
13. Kaushal B, Chauhan S, Magoon R, Krishna NS, Saini K, Bhoi D, et al. Efficacy of bilateral erector spinae plane block in management of acute postoperative surgical pain after pediatric cardiac surgeries through a midline sternotomy. J Cardiothorac Vasc Anesth. 2020; 34:981–6.

14. Macaire P, Ho N, Nguyen V, Phan Van H, Dinh Nguyen Thien K, Bringuier S, et al. Bilateral ultrasound-guided thoracic erector spinae plane blocks using a programmed intermittent bolus improve opioid-sparing postoperative analgesia in pediatric patients after open cardiac surgery: a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2020; 45:805–12.

15. Voulgarelis S, Halenda GM, Tanem JM. A novel use of liposomal bupivacaine in erector spinae plane block for pediatric congenital cardiac surgery. Case Rep Anesthesiol. 2021; 2021:5521136.

16. Abdelbaser II, Mageed NA. Analgesic efficacy of ultrasound guided bilateral transversus thoracis muscle plane block in pediatric cardiac surgery: a randomized, double-blind, controlled study. J Clin Anesth. 2020; 67:110002.

17. Cakmak M, Isik O. Transversus thoracic muscle plane block for analgesia after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2021; 35:130–6.

18. Zeballos JL, Voscopoulos C, Kapottos M, Janfaza D, Vlassakov K. Ultrasound-guided retrolaminar paravertebral block. Anaesthesia. 2013; 68:649–51.

19. Damjanovska M, Stopar Pintaric T, Cvetko E, Vlassakov K. The ultrasound-guided retrolaminar block: volume-dependent injectate distribution. J Pain Res. 2018; 11:293–9.

20. Nobukuni K, Hatta M, Nakagaki T, Yoshino J, Obuchi T, Fujimura N. Retrolaminar versus epidural block for postoperative analgesia after minor video-assisted thoracic surgery: a retrospective, matched, non-inferiority study. J Thorac Dis. 2021; 13:2758–67.

21. Sotome S, Sawada A, Wada A, Shima H, Kutomi G, Yamakage M. Erector spinae plane block versus retrolaminar block for postoperative analgesia after breast surgery: a randomized controlled trial. J Anesth. 2021; 35:27–34.

Fig. 4.
Postoperative modified objective pain score (MOPS). Values are presented as median (Q1, Q3). The thick black line in the boxplot represents the median, the upper border represents Q3 and the lower border represents Q1. *P < 0.05 and †P < 0.001 vs. TRLB group.
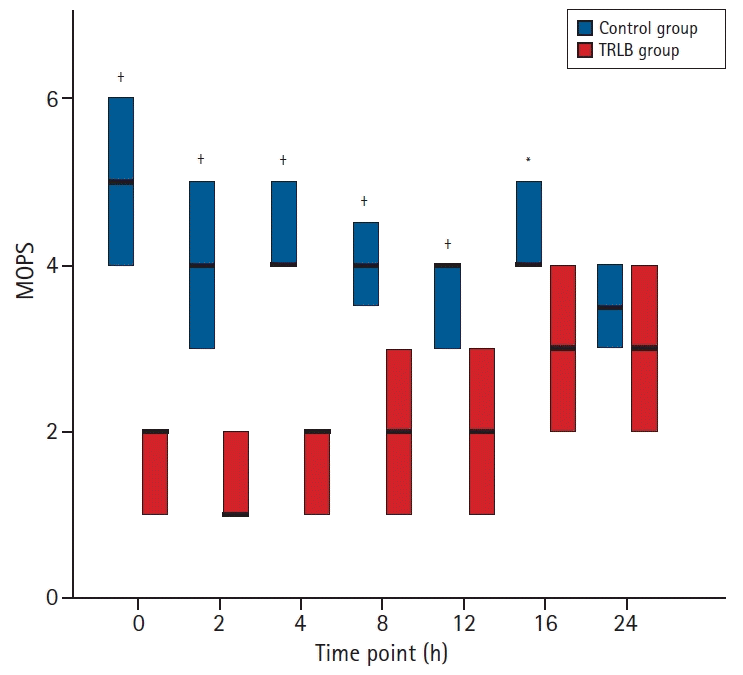
Table 1.
Patients and Surgical Characteristics
Table 2.
Intraoperative Fentanyl Consumption and Postoperative Variables




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download