This article has been
cited by other articles in ScienceCentral.
Abstract
Prompt, effective treatment is necessary following aneurysmal subarachnoid hemorrhage to prevent recurrent rupture, which is thought to double mortality. Atypical ruptured aneurysms, such as blister or dissecting pseudoaneurysms, or those that are unusually distal in the middle cerebral artery (MCA) are challenging to treat with either open or endovascular options, though the pipeline embolization device (PED) has shown promise in multiple case series. We present a case of a ruptured dissecting pseudoaneurysm in the distal MCA (distal M3/proximal M4) prefrontal division in an healthy young patient (<60 years) successfully treated with a PED. The PED was chosen both as the only vessel sparing option in the young patient as well as for its potential as a vessel sacrifice tool if the pseudoaneurysm was felt to be incompletely treated, which in this case was not necessary—though would have leveraged the thrombogenicity of the device as a therapeutic advantage.
Keywords: Pseudoaneurysm, Subarachnoid hemorrhage, Embolization, therapeutic, Middle cerebral artery
INTRODUCTION
Spontaneous subarachnoid hemorrhage (SAH) is a potentially devastating neurological event with an associated mortality rate of 25% within the first 24 hours [
1]. The overwhelming majority of aneurysms are located on the circle of Willis or proximal anterior or posterior distributions and are saccular in etiology [
2]. Less common types of aneurysms, such as those in the distal middle cerebral or anterior cerebral locations or those with an unusual morphology, are often more complex to treat [
3]. Currently, there is no standard of treatment available for distal or dissecting aneurysms.
Increasingly, flow diversion devices, such as the pipeline embolization device (PED), have been utilized in the treatment of dissecting aneurysms [
4]. The PED was originally developed for large unruptured aneurysms in the anterior circulation [
5,
6], but has become a promising treatment option for ruptured and/or wide neck and fusiform aneurysms [
7].
We present a case in which a younger patient with a likely dissecting aneurysm in the distal left middle cerebral artery (MCA) was successfully treated with flow diversion. This is the most distal use of the PED to our knowledge, and the excellent technical and clinical outcomes are instructive.
CASE REPORT
Presentation
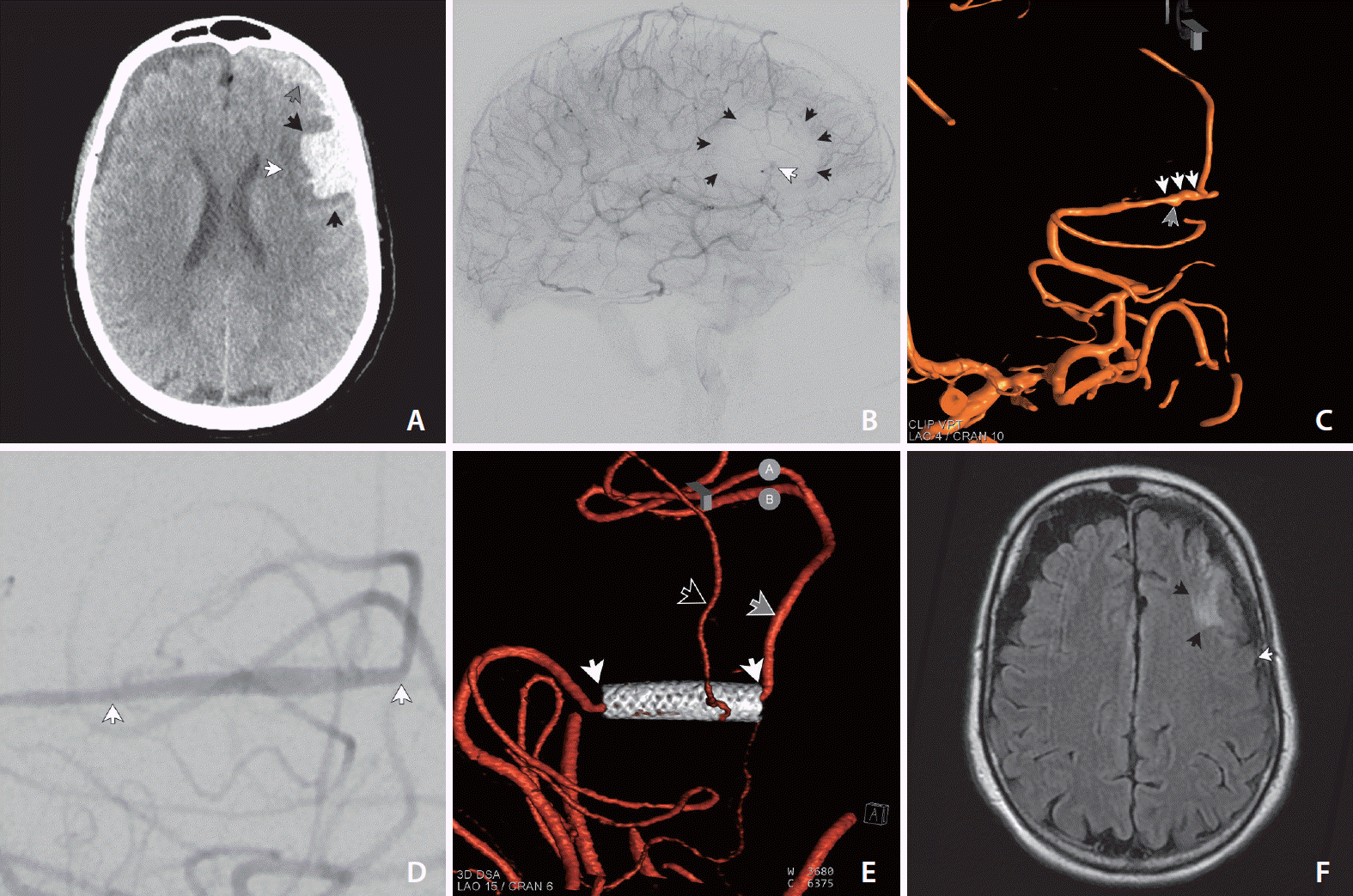
A patient (<60 years old) with a past medical history of anxiety, depression, fibromuscular dysplasia, and breast cancer treated with lumpectomy and radiation in 2014–2015 was admitted to an outside hospital in early December 2019 with headache, neck pain, and vomiting. At this time, the patient was alert and oriented with intact speech. A computed tomography (CT) scan (
Fig. 1A) demonstrated SAH and parenchymal hemorrhage focused on the left superior and middle frontal gyrus in the prefrontal MCA distribution (
Fig. 1). In addition, there was subdural hemorrhage. A catheter angiogram was performed and was consistent with dissecting pseudoaneurysm within the prefrontal division of the left MCA at the level of M3/M4, and this artery traversed the center of the collection of parenchymal and SAH (
Fig. 1B). Five days after symptom onset, the patient was transferred to our hospital for endovascular intervention. On exam, the patient was alert and oriented and reported having a headache, but was negative for neurological deficits.
Catheter angiography showed a left MCA dissecting pseudoaneurysm in the M3 segment over the course of an 8 mm length of the artery with a maximum dimension of 2 mm and a minimum arterial diameter of slightly less than 1 mm (
Fig. 1C). After a discussion of various treatment options, largely revolving around forms of arterial sacrifice, the decision was made to attempt to spare the artery using a PED. The plan was that if the implant looked ineffective in any way—for example, if the dissecting pseudoaneurysm continued to opacify briskly (or enlarge) or if the luminal diameter appeared too constrained following PED implant to continue to act as an effective conduit for antegrade flow, then the patient would be taken off their antiplatelet regimen and the highly thrombogenic PED would be used as an arterial sacrifice device.
Procedure
The patient was given 90 mg of ticagrelor at 4 AM and 90 mg of ticagrelor at 6 AM with 81 mg of aspirin for an 8 AM procedure start time as per our practice protocol for ruptured flow diversion cases. Maintenance thereafter was with 60 mg of ticagrelor twice daily and 81 mg of aspirin. The case was performed on a Siemens Artis Q biplane unit (Siemens, Munich, Germany). Femoral arterial access was utilized, and a 90 cm Neuron Max (Penumbra, Alameda, CA, USA) was utilized as a guide sheath. An 0.044-inch Phenom Plus (Medtronic, Irvine, CA, USA) was utilized as an intermediate catheter. All arteriography was performed through the Phenom Plus. Given the degree of vasospasm noted during routine catheterization, 5 mg of verapamil was given as premedication into the internal carotid artery, though this is not part of our practice routine.
Distal arterial access was achieved using a Phenom 27 microcatheter (Medtronic) advanced over a 0.014-inch Traxcess guidewire (MicroVention, Tustin, CA, USA). As per our practice routine, intravenous heparin (5,000 U intravenous bolus) was administered after catheter access across the segmental pseudoaneurysm.
Following a latency of 4 minutes, a 2.5×10 mm PED was deployed across the lesion with the distal aspect within the proximal M4 segment and proximally terminating within the M3 segment, spanning the dissecting segment with 3–4 mm landing zones on either zone. Left internal carotid arteriography demonstrated mild vasospasm within the prefrontal M3 division and stent patency with normal antegrade flow, and there was no longer visualization of the pseudoaneurysm (image not shown).
Another left internal carotid arteriographic run was performed following a latency of 310 minutes that showed stent patency and normal antegrade flow as well as decreased vasospasm (
Fig. 1D). The decision was made to continue the patient’s dual antiplatelets and bring the patient back later in the day to see if the stent remained widely patent and if the pseudoaneurysm remained stable.
Post Procedure
The patient tolerated the procedure well and post-operatively remained neurologically stable, following commands. The patient was maintained on nimodipine, levetiracetam, and a statin as per SAH protocol. Catheter angiography later the same day demonstrated stent patency and excellent antegrade flow with normal arterial transit (image not shown). There was no visualization of the pseudoaneurysm, and there was minimal vasospasm distal to the PED. The decision was made to continue the antiplatelet regimen to preserve the artery. A CT done 3 days postoperatively showed a stable left frontal opercular intraparenchymal hemorrhage and significant improvement of the SAH. Transcranial dopplers were negative for vasospasm. Magnetic resonance angiography 5 days post-procedure demonstrated a normal flow-related signal within the stent suggesting persistent normal antegrade flow. There were stable left frontal parenchymal and subdural hematomas (image not shown).
The patient experienced transient episodes of encephalopathy twice during the course of recovery without focality characterized by mild word-finding difficulty and intermittent headaches as well as mild paresthesia in the left foot, which was unchanged from the pre-procedure baseline. On the fifteenth postoperative day, the patient was sent home with outpatient physical and occupational therapy.
Follow Up
The patient came in for follow-up catheter cerebral angiography 6 months after the procedure (
Fig. 1E). Left internal carotid arteriography demonstrated good patency of the PED, as well as antegrade flow of the M4 branch that had been jailed by the stent. Interestingly, in the interim, the pipeline stent which had previously been constrained within less than 1 millimeter diameter of the artery, had expanded to its nominal diameter of 2.5 mm. The entire lumen of the 2.5 mm device opacified with contrast resulting in a segment of the ectatic reconstructed artery that otherwise appeared normal without residual luminal irregularity or pseudoaneurysm. Follow-up magnetic resonance imaging (MRI) demonstrated encephalomalacia limited to the original region of the parenchymal hemorrhage, not involving the territory of the dominant division of the prefrontal branch that supplied the territory posterior to the region of post-hemorrhage encephalomalacia (
Fig. 1F).
DISCUSSION
This case is clinically significant for a multitude of reasons. First, this study provides further evidence for the efficacy of endovascular flow diversion with the PED in treating ruptured aneurysms. The use of a PED for the treatment of ruptured aneurysms with architecture that makes conventional saccular approaches (surgical clip reconstruction, endovascular coiling, Woven EndoBridge embolization [MicroVention, Aliso Viejo, CA, USA]) technically challenging or high risk is becoming increasingly prevalent because of the inherent advantages of the endoluminal approach in these cases and the empirically excellent clinical outcomes [
7,
8]. This patient’s aneurysm was unique in that it was likely a ruptured dissecting aneurysm as well as very distal. While there have been reports of PED use to treat dissecting aneurysms, to our knowledge there has not been a case of the PED for treatment of an aneurysm in such a distal location. The alternative and more conventional approach would have been arterial sacrifice, likely with coils; though, completely occluding pial arteries with coils can be challenging, and attempting curative coil occlusion in a dissecting aneurysm carries an inherent risk of perforation. Our approach was also designed to spare as much cortex as possible, and while the territory spared is not traditionally considered eloquent, there are benefits to minimizing cortical gliosis in a younger patient, such as lowering the likelihood of developing a chronic seizure disorder [
9]. Follow-up MRI in this case clearly demonstrated intact cortex and subcortical white matter dorsal to the area of parenchymal hemorrhage in the territory supplied by the dominant branch of the prefrontal division of the MCA.
While there is an inherent risk in using flow diversion in very-small caliber vessels, a recent case series of 67 patients demonstrated the use of flow diverters in small arteries in a caliber of less than 2 mm, resulting in only 1 case of flow delay associated with flow diameter reduction [
10]. In this case, The artery remained patent and expanded with the device as evidenced by the ectasia within the stented segment when compared to the native artery immediately proximal and distal to the PED implant, which suggests that the relatively thin-walled distal pial arterial circulation is amenable to flow diversion and is tolerant of oversized devices over time.
Another application from this case is the use of the PED’s thrombogenicity as a potential therapeutic strategy. As mentioned, if the segment containing the PED implant was not effective in treatment of the pseudoaneurysm, the strategy was to use the PED as a closure device, taking advantage of the thrombogenicity of a braided metal implant. While this strategy was unnecessary in this patient’s case, it could provide an alternative course of action when treating distal arterial aneurysms.
This case showed the effectiveness of flow diversion in treating ruptured dissecting aneurysms as well as for treating aneurysms complicated by distal location. The device’s thrombogenicity should be considered as a therapeutic strategy in the appropriate clinical circumstances.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download