Abstract
A male in his 60s presented with transient ischemic attacks 5 years after aortic arch branch graft repair for type A aortic dissection. Computed tomographic angiography demonstrated 80% stenosis of the brachiocephalic artery close to the origins of the right common carotid and subclavian arteries. The case was reviewed at our multidisciplinary aortic meeting and a plan for endovascular management was made. Percutaneous endovascular Y stenting from the innominate artery into the left common carotid and subclavian arteries was achieved using self-expanding nitinol stents with a rendezvous technique that included retrograde right radial artery, retrograde right external carotid artery, and retrograde right femoral arterial approaches. At 6 months review, the stents remained widely patent and the patient was symptom-free.
Extracranial carotid occlusive disease is a common stroke mechanism, accounting for 7–20% of all first-time ischemic strokes [1,2]. Endovascular treatment of aortic branch origin disease is safe and efficacious [3] with low restenosis rates up to 12 months post-treatment and low rates of ipsilateral stroke [4]. Although long-term data suggest increasing restenosis rates [5], recurrent symptomatic lesions can be safely treated via an endovascular approach [3]. There is a paucity of studies and appropriate treatment options regarding carotid stenting and recanalization of stenoses in the setting of aortic arch branch reconstruction.
We present a case describing a novel combined Y stent of the innominate artery, right subclavian, and right common carotid artery (CCA) using wire rendezvous techniques from radial, carotid, and femoral approaches.
A male in his 60s presented 2 months before the Y stenting procedure with symptoms of left middle cerebral artery (MCA) stroke. He had a past history of type A aortic dissection 5 years earlier treated with subtotal aortic arch replacement including a branched graft to the innominate and left common carotid arteries.
Computed tomography angiogram (CTA) angiogram on presentation showed occlusion of the native left CCA along with the left carotid limb of the branched graft. The left internal and external carotid arteries remained patent consistent with retrograde filling from the external to internal carotid. Left M1 MCA occlusion was also present. The patient underwent successful endovascular retrieval of the M1 embolus via a direct internal carotid artery puncture. Thrombolysis in cerebral infarction 3 flow was restored, and the patient made a complete recovery. A decision was made not to attempt recanalization of the occluded left CCA and left common carotid graft limb.
A subsequent review of serial CTA imaging indicated that the patient had progressive high-grade stenosis at the anastomosis between the graft and brachiocephalic trunk associated with extensive soft tissue density in the surrounding mediastinum. A biopsy of this tissue revealed reactive fibrosis and inflammatory change but no evidence of tumor or infection. On further questioning, the patient described recurrent episodes of right-sided visual symptoms over 3 months which persisted following stroke intervention and were diagnosed by a stroke neurologist as transient retinal ischemia.
Due to extensive untreatable occlusive disease of the left CCA seen during mechanical thrombectomy, incidental severe stenosis of the innominate artery, and micro-embolic symptoms, the decision, following multidisciplinary meeting consensus, was to perform endovascular stenting on the right side to maintain cerebral perfusion.
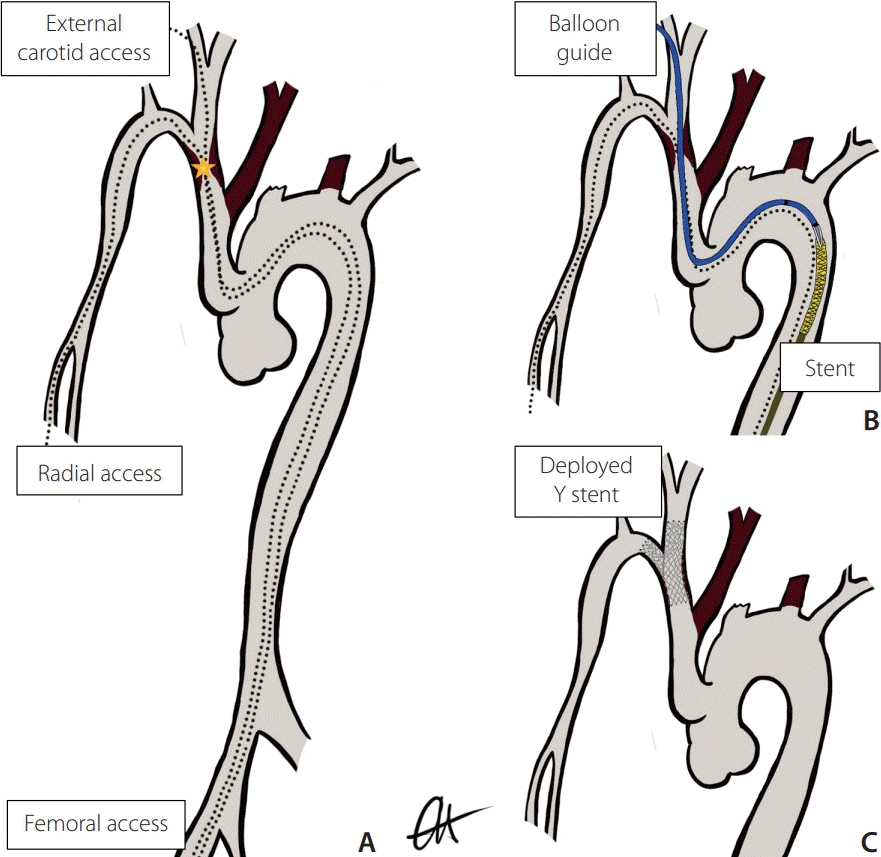
The procedure was performed with the patient under general anesthesia with intravenous heparin 7,000 IU administered at the start of the procedure. No intra-operative antibiotics were administered. The patient was positioned such that simultaneous access to the right common femoral artery (CFA), radial artery (RA), and external carotid artery (ECA) could be obtained. A concept diagram demonstrates the anatomy and expected progression in a theater (Fig. 1).
Arterial access was obtained under ultrasound guidance as follows: right radial arterial access at the right anatomical snuffbox with placement of a 6 French Slender radial arterial sheath introducer (Terumo, Tokyo, Japan), right ECA puncture close to the carotid bifurcation with insertion of a 4 French sheath introducer (Terumo), and right CFA puncture with insertion of a 9 French introducer sheath (Terumo).
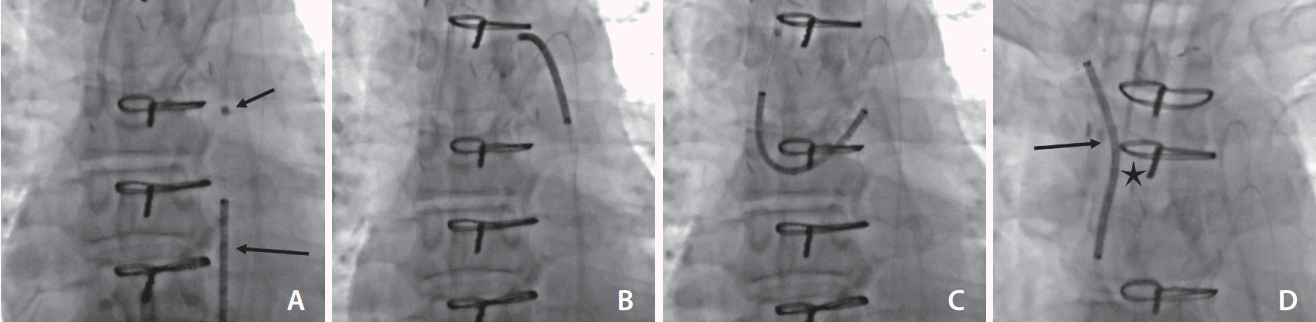
A 6 French Envoy DA guiding catheter (Codman Neuro, Raynham, MA, USA) was advanced over a 180 cm length 0.018-inch guidewire (Glidewire; Terumo) from the radial access into wire into the right subclavian artery. A 6 French Neuron Max catheter (Penumbra Inc., Alameda, CA, USA) and a 6 French Envoy DA guiding catheter (Codman Neuro) were advanced from the femoral access to the level of the aortic arch over a 180 cm length 0.014-inch guidewire (Glidewire Advantage; Terumo) wire. High-grade stenosis of the innominate artery was confirmed and noted at the time of the catheter digital subtraction angiography (DSA).
Subsequently, 300 cm length 0.014-inch Synchro Standard wires (Stryker Neuro, Fremont, CA, USA) were advanced via both the right ECA and radial access sites to cross the innominate stenosis. Through the femoral access sheath, a 6 French CloverSnare® 4-Loop Vascular Retriever (Cook Medical, Bloomington, IN, USA) was positioned in the aortic arch and snare capture of Synchro wires was achieved, resulting in a through and through carotid-femoral and radial-femoral access.
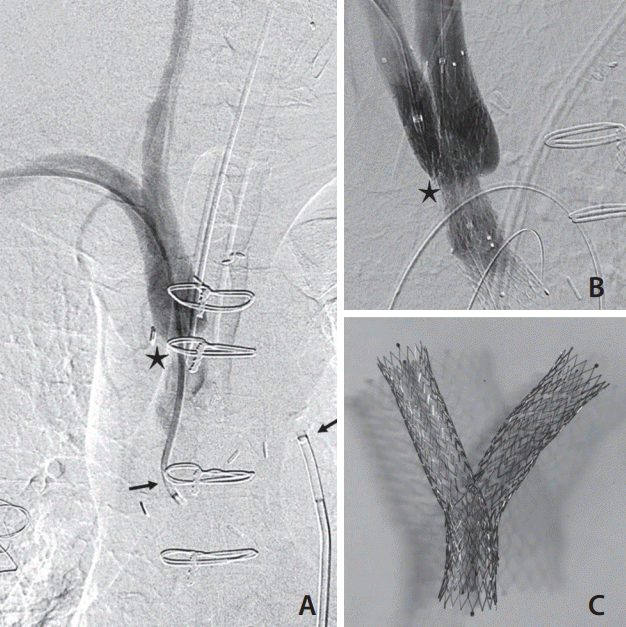
A Venovo® Venous stent, 14×40 mm (diameter×length) (Bard, Covington, GA, USA) was advanced from the femoral access site over the RA wire to stent from the innominate into the subclavian artery. The Venovo® system was utilized due to its relative stiffness and rigidity, in addition to the inherent larger sizing of the target vessel (>10 mm) and the ability to manipulate and construct a Y stent ex-vivo. To improve the transition between the 0.035-inch delivery device and 0.014-inch wire, a 4×8 mm Coyote™ balloon (Boston Scientific, Marlborough, MA, USA) was introduced through the radial access to enter the tip of the Venovo delivery catheter in the proximal aorta. This apposition between stent and balloon, whereby the balloon tapered tip would fit within the tapered tip of the stent, allowed seamless transition and migration of the 0.035-inch device across the 0.014-inch wire. The stent device was then advanced into the branch graft and across the stenosis with gentle traction of both wire ends to support the transition (Fig. 2). To fashion the carotid limb of the “Y stent”, a Progreat® 2.4 French microcatheter and 0.018-inch Glide wire (Terumo) were advanced retrogradely from the right external carotid access through the wall of the Venovo graft. Snare retrieval with a 6 French CloverSnare® 4-Loop Vascular Retriever (Cook Medical) was once again performed resulting in a through and through wire system crossing the carotid/innominate stenosis. Balloon dilatation across the stent wall was performed using a Mustang™ 9.0×40 mm balloon dilatation catheter (Boston Scientific) to increase the access site for being deployed stent (Venovo® Venous stent, 12×60 mm [diameter×length] [Bard]). Balloon dilatation of the crossed stent was performed using an Armada™ 35 10×40 mm balloon dilatation catheter (Abbot, Abbott Park, IL, USA). Completion angiogram demonstrated a widely patent innominate artery with free flow through the carotid and subclavian artery. No embolic complication was identified on cerebral imaging. Fig. 3 shows the pre-and post-treatment DSA in addition to the stent constructed ex-vivo.
Hemostasis was achieved using 6-French and 8-French Angio-seal devices (Terumo) for the ECA and femoral punctures, respectively, and a Precludesync distal™ radial compression device (Merit Medical, South Jordan, UT, USA) for the right radial puncture.
The patient recovered well from the procedure with a continuation of dual antiplatelet therapy and was discharged 4 days post-procedure. The stent remained widely patent on CT at 3 months, and the patient was well with no neurological symptoms at the 6-month review.
Addressing symptomatic CCA disease is important due to the significant morbidity linked with increased ischemic stroke [2]. Aortic arch reconstruction surgery provides additional challenges in the endovascular management of these stenoses. Through and through access provides improved control allowing safe and accurate maneuvering and positioning of devices that would otherwise be difficult or impossible to deploy through such tortuous anatomy. Given the required dimensions of the stent, in addition to the tortuosity of the post-operative aorta, the authors considered it impractical and unsafe to traverse the treated stenosis and deploy a stent without uphill/proximal guidance and control. This technique not only provides greater stability than conventional inline stent deployment, but also allows options for damage control in the unlikely event of arterial rupture given the presence of long length wire stability.
The use of direct carotid access proved useful and necessary in this intervention as the authors required proximal or upstream control and access. The concern with any arterial intervention is hemorrhage, and as such, careful vascular closure is needed. An ECA puncture away from the carotid bulb also obviates the risk of baroreceptor-mediated hypotension and bradycardia.
While 0.014-inch microwires allow easy manipulation through tortuous anatomy and stent interstices, the addition of a 0.014-inch balloon catheter provides a smooth transition between the 0.014-inch microwires and the 0.035-inch instent delivery system during device navigation. This theoretically reduces the risk of distal embolization associated with the ‘snowplow effect’ as the stent is advanced antegrade across the stenosis.
Endovascular Y stenting of innominate stenosis is technically feasible. In this case, it was performed to avoid the need for complex re-do surgery. Careful planning is important in such cases. A potential difficulty with complex anatomy can be minimized using a through and through wire technique. We also introduce the concept of a balloon in catheter technique to improve transitions between devices and wires.
Notes
REFERENCES
1. White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among Whites, Blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005; 111:1327–1331.

2. Veith FJ, Amor M, Ohki T, Beebe HG, Bell PR, Bolia A, et al. Current status of carotid bifurcation angioplasty and stenting based on a consensus of opinion leaders. J Vasc Surg. 2001; 33(2 Suppl):S111–S116.

3. van de Weijer MA, Vonken EJ, de Vries JP, Moll FL, Vos JA, de Borst GJ. Technical and clinical success and long-term durability of endovascular treatment for atherosclerotic aortic arch branch origin obstruction: evaluation of 144 procedures. Eur J Vasc Endovasc Surg. 2015; 50:13–20.

4. Clavel P, Hebert S, Saleme S, Mounayer C, Rouchaud A, Marin B. Cumulative incidence of restenosis in the endovascular treatment of extracranial carotid artery stenosis: a meta-analysis. J Neurointerv Surg. 2019; 11:916–923.

5. Bonati LH, Gregson J, Dobson J, McCabe DJH, Nederkoorn PJ, van der Worp HB, International Carotid Stenting Study investigators, et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): secondary analysis of a randomised trial. Lancet Neurol. 2018; 17:587–596.
Fig. 1.
Concept sketch of the procedural plan. Image (A) confirms ECA/radial and femoral access with wires (dotted lines) crossing the stenosis (★) of the innominate branch graft. Image (B) imaging demonstrates the monorail/chaperone system of the balloon catheter and stent device. Image (C) shows the expected position and appearance of the Y stent configuration. ECA, external carotid artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download