INTRODUCTION
Table 1.
| Indications | |
|---|---|
| Within 3 hours† (COR I) * | • Age ≥18 years |
| • NIHSS score ≥6 | |
| 3 to 4.5 hours† (COR I)* | • Age ≥18 years and ≤80 years |
| • Without a history of both diabetes mellitus and prior stroke | |
| • NIHSS score ≥6 and ≤25 | |
| • Not taking any OACs | |
| • Without imaging evidence of ischemic injury involving more than one-third of the MCA territory: IVT is recommended in the setting of early ischemic changes on NCCT of mild to moderate extent (other than frank hypodensity) | |
| Wake-up stroke or unclear time of onset >4.5 hours from last known normal state or at baseline state (COR IIa)* | IVT can be beneficial when DWI lesion is smaller than one-third of the MCA territory and no visible signal change on FLAIR |
| CMB (COR IIa)* | • Eligible patients who have previously had a small number (1–10) of CMBs demonstrated on MRI, IVT is reasonable |
| • Eligible patients who have previously had a high burden of CMBs (>10) demonstrated on MRI, IVT may be associated with an increased risk of symptomatic ICH, and the benefits of treatment are uncertain. IVT may be reasonable if there is the potential for substantial benefit | |
| Contraindication | |
| • NIHSS score 0–5 (COR III: no benefit)* | |
| • NCCT: extensive regions of frank hypodensity (COR III: no benefit)* | |
| • Acute ICH (COR III: harm)* | |
| • Ischemic stroke within 3 months (COR III: harm)* | |
| • Severe head trauma within 3 months (COR III: harm)* | |
| • Posttraumatic infarction that occurs during the acute in-hospital phase (COR III: harm)* | |
| • Intracranial/intraspinal surgery within 3 months (COR III: harm)* | |
| • History of ICH (COR III: harm)* | |
| • Symptoms and signs most consistent with a subarachnoid hemorrhage (COR III: harm)* | |
| • Gastrointestinal malignancy (COR III: harm)* | |
| • Gastrointestinal bleeding within 21 days (COR III: harm)* | |
| • Coagulopathy: platelet count <100,000/mm3, INR >1.7, aPTT >40 s, or PT >15 s (COR III: harm)* | |
| • Infective endocarditis (COR III: harm)* | |
| • Aortic arch dissection (COR III: harm)* | |
| • Intra-axial intracranial neoplasm (COR III: harm)* | |
| • Full treatment dose of LMWH within the previous 24 hours (COR III: harm)* | |
| • Patients taking direct thrombin inhibitors or direct factor Xa inhibitors (COR III: harm)* | |
| • Abciximab should not be administered concurrently with IV rt-PA (COR III: harm)* | |
| • IV aspirin should not be administered within 90 minutes after the start of IV rt-PA (COR III: harm)* | |
AHA, American Heart Association; ASA, American Stroke Association; COR, class of recommendation; MRI, magnetic resonance imaging; rt-PA, recombinant tissue plasminogen activator; IV, intravenous; aPTT, activated partial prothrombin time; CMB, cerebral microbleed; DWI, diffusion-weighted magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; ICH, intracranial hemorrhage; INR, international normalized ratio; IVT, intravenous thrombolysis; LMWH, low-molecular-weight heparin; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; NIHSS, National Institutes of Health Stroke Scale; OAC, oral anticoagulant; PT, prothrombin time.
* COR amended to conform with American College of Cardiology/AHA 2015 Recommendation Classification System: COR I (strong), benefit>>>risk; COR IIa (moderate), benefit>>risk; COR IIb (weak), benefit≥risk; COR III (no benefit), benefit=risk; COR III (harm), risk>benefit. [8]
Table 2.
| Indications | |||
|---|---|---|---|
| Within 6 hours† (COR I)* | • Prestroke mRS score of 0 to 1 | ||
| • Causative occlusion of the ICA or MCA segment 1 (M1) | |||
| • Age ≥18 years | |||
| • NIHSS score ≥6 | |||
| • ASPECTS of ≥6 | |||
| • Treatment can be initiated (groin puncture) within 6 hours of symptom onset | |||
| Within 6 hours† (COR IIb)* | • Causative occlusion of the MCA segment 2 (M2) or MCA segment 3 (M3) | ||
| • mRS score >1, ASPECTS <6, or NIHSS score <6, and causative occlusion of the ICA or M1 | |||
| • Causative occlusion of the anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries | |||
| Concomitant with IVT (COR I)* | • Patients eligible for IVT should receive IV rt-PA even if EVT is being considered (COR I)* | ||
| • In patients under consideration for EVT, observation after IVT to assess for clinical response should not be performed (COR III: harm)* | |||
| 6 to 16 hours of last known normal state (COR I)* | • Follow DAWN12 or DEFUSE 313 eligibility criteria | ||
| • DAWN eligibility criteria | |||
| - 6–24 hours after last known normal state | |||
| - Age ≥18 years | |||
| - NIHSS score ≥10 | |||
| - Prestroke mRS score of 0 or 1 | |||
| - Infarct involving <1/3 MCA territory by CT or DWI | |||
| - Occlusion of the ICA and/or MCA (M1) | |||
| - Mismatch between the severity of the clinical deficit and the infarct volume | |||
| A. Age ≥80 years, NIHSS score ≥10 and infarct volume <21 mL | |||
| B. Age <80 years, NIHSS score ≥10 and infarct volume <31 mL | |||
| C. Age <80 years, NIHSS score ≥20 and infarct volume <51 mL | |||
| • DEFUSE 3 eligibility criteria | |||
| - 6–16 hours after last known normal state | |||
| - Age 18–90 years | |||
| - NIHSS score ≥6 | |||
| - Prestroke mRS score ≤2 | |||
| - Infarct involving <1/3 MCA territory by CT or DWI | |||
| - Occlusion of the ICA and/or MCA (M1) | |||
| - Imaging criteria | |||
| A. Volume of core <70 mL | |||
| B. Ratio of volume of penumbra to core >1.8 | |||
| C. Volume of penumbra >15 mL | |||
| 16 to 24 hours of last known normal state (COR IIa)* | • Follow DAWN12 eligibility criteria | ||
AHA, American Heart Association; ASA, American Stroke Association; EVT, endovascular thrombectomy; COR, class of recommendation; mRS, modified Rankin Scale; rt-PA, recombinant tissue plasminogen activator; IV, intravenous; ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; DWI, diffusion-weighted magnetic resonance imaging; ICA, internal carotid artery; IVT, intravenous thrombolysis; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale.
* COR amended to conform with American College of Cardiology/AHA 2015 Recommendation Classification System: COR I (strong), benefit>>>risk; COR IIa (moderate), benefit>>risk; COR IIb (weak), benefit≥risk; COR III (no benefit), benefit=risk; COR III (harm), risk>benefit. [8]
Table 3.
| Brain imaging | COR* | New, revised, or unchanged | |
|---|---|---|---|
| Initial imaging | |||
| 1. All patients with suspected acute stroke should receive an emergency brain imaging evaluation on first arrival to a hospital before initiating any specific therapy to treat AIS | I | Reworded for clarity from 2013 AIS guidelines† | |
| 2. Systems should be established so that brain imaging studies can be performed as quickly as possible in patients who may be candidates for IVT or EVT or both | I | Revised from 2013 guidelines† | |
| 3. NCCT is effective to exclude ICH before IVT | I | Revised from 2013 guidelines† | |
| 4. MRI is effective to exclude ICH before IVT | I | New recommendation | |
| 5. CTA with CTP or MRA with DWI with or without MRP is recommended for certain patients | |||
| IVT eligibility | |||
| 1. Administration of rt-PA in eligible patients without first obtaining MRI to exclude CMBs is recommended | I | New recommendation | |
| 2. In patients eligible for IVT, because the benefit of therapy is time-dependent, treatment should be initiated as quickly as possible and not delayed for additional multimodal neuroimaging, such as CTP and MRP | I | New recommendation | |
| 3. In patients with AIS who awoke with stroke symptoms or have unclear time of onset >4.5 hours from last known normal state or at baseline state, MRI to identify DWI-positive FLAIR- negative lesions can be useful for selecting those who can benefit from IVT within 4.5 hours of stroke symptom recognition | IIa | New recommendation | |
| EVT eligibility-vessel imaging | |||
| 1. For patients who otherwise meet criteria for EVT, noninvasive vessel imaging of the intracranial arteries is recommended during the initial imaging evaluation | I | Reworded for clarity from 2015 endovascular‡ | |
| 2. For patients with suspected LVO who have not had noninvasive vessel imaging as part of their initial imaging assessment for stroke, noninvasive vessel imaging should then be obtained as quickly as possible (e.g., during rt-PA infusion if feasible) | I | Revised from 2015 endovascular‡ | |
| 3. In patients with suspected intracranial LVO and no history of renal impairment, who otherwise meet criteria for EVT, it is reasonable to proceed with CTA if indicated before obtaining a serum creatinine concentration | IIa | New recommendation | |
| 4. In patients who are potential candidates for EVT, imaging of the extracranial carotid and vertebral arteries, in addition to the intracranial circulation, may be reasonable to provide useful information on patient eligibility and endovascular procedural planning | IIa | New recommendation | |
| 5. It may be reasonable to incorporate collateral flow status into clinical decision-making in some candidates to determine eligibility for EVT | IIb | Revised from 2015 endovascular‡ | |
| EVT eligibility-multimodal imaging | |||
| 1. When selecting patients with AIS within 6 to 24 hours of last known normal status who have LVO in the anterior circulation, obtaining CTP or DWI, with or without MRP, is recommended to aid in patient selection for EVT, but only when patients meet other eligibility criteria from one of the RCTs that showed benefit from EVT in this extended time window | I | New recommendation | |
| 2. When evaluating patients with AIS within 6 hours of last known normal status with LVO and an ASPECTS of ≥6, selection for EVT based on CT and CTA or MRI and MRA is recommended compared to performance of additional imaging such as CTP or MRP | I | New recommendation | |
AHA, American Heart Association; ASA, American Stroke Association; COR, class of recommendation; rt-PA, recombinant tissue plasminogen activator; AIS, acute ischemic stroke; ASPECTS, Alberta Stroke Program Early CT Score; CMB, cerebral microbleed; CT, computed tomography; CTA, computed tomography angiography; CTP, computed tomography perfusion imaging; DWI, diffusionweighted magnetic resonance imaging; EVT, endovascular thrombectomy; FLAIR, fluid attenuated inversion recovery; ICH, intracranial hemorrhage; IVT, intravenous thrombolysis; LVO, large vessel occlusion; MR, magnetic resonance; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MRP, magnetic resonance perfusion imaging; NCCT, noncontrast computed tomography; RCT, randomized clinical trial.
* COR amended to conform with American College of Cardiology/AHA 2015 Recommendation Classification System: COR I (strong), benefit>>>risk; COR IIa (moderate), benefit>>risk; COR IIb (weak), benefit≥risk; COR III (no benefit), benefit=risk; COR III (harm), risk>benefit. [8]
Assessment of Brain Parenchyma
Intracranial hemorrhage
Core
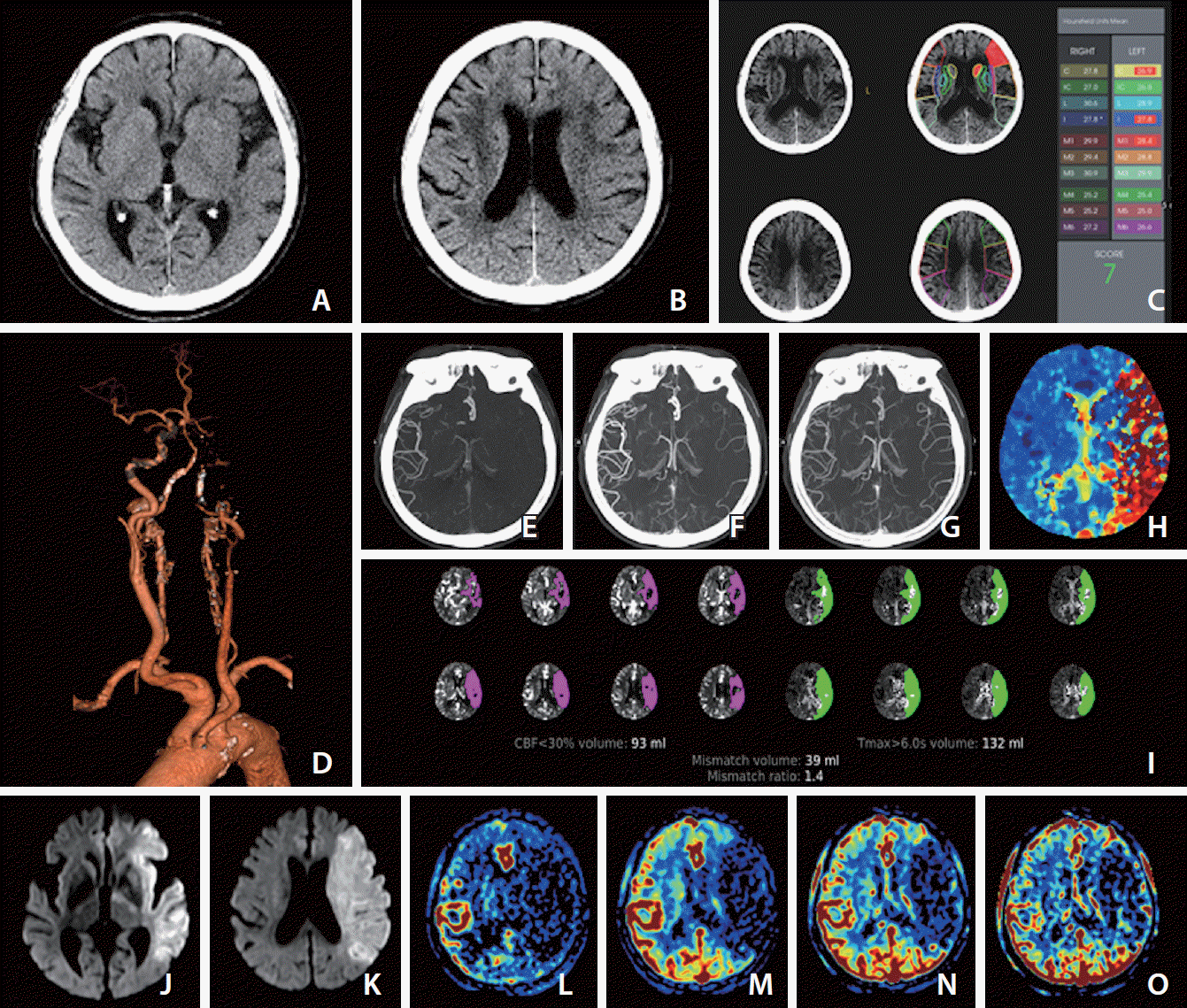 | Fig. 1.Images at admission of an elderly patient who presented with dysarthria and right-sided weakness at 2 and half hours after last known normal state. The premorbid modified Rankin scale score of this patient was 0, and the National Institutes of Health Stroke Scale score at admission was 23. (A) Ganglionic level and (B) supraganglionic level, noncontrast computer tomography (NCCT) images show loss of gray-white matter differentiation at insula, M2, M3, M4, and M5 of the left middle cerebral artery territory, and the Alberta Stroke Program Early CT Score (ASPECTS) is 5 visually. (C) Computer-aid automated ASPECTS is 7 (RAPID, RapidAI®, Menlo Park, CA, USA). (D) Computed tomography angiography (CTA) shows occlusion of the left internal carotid and middle cerebral arteries. (E–G) The first (E), second (F), and third phase (G) collateral images derived from multiphase CTA show a filling delay of 2 phases in the affected hemisphere with a significantly reduced number of vessels in the ischemic territory (multiphase CTA collateral score 2). (H) CT perfusion (CTP) Tmax threshold >6 seconds (Tmax >6 s) map indicates near whole left middle cerebral artery territory as penumbra (red zone). (I) Automated software (RAPID, RapidAI®) demonstrates the volume data of core and penumbra and mismatch ratio between the penumbra and core, which are 93 mL with a threshold of CTP cerebral blood flow <30% and 132 mL with threshold of a Tmax > 6 s and 1.4, respectively. (J) Ganglionic level and (K) supraganglionic level, diffusion-weighted magnetic resonance images (DWI) obtained 10 minutes after CT scan show a large core involving the entire left middle cerebral artery territory. (L–O) Images of arterial (L), capillary (M), early venous (N), and late venous (O) phases of the collateral map derived from dynamic contrast-enhanced magnetic resonance angiography show very poor collateral perfusion status (MAC score of 0) [38] defined as collateral perfusion delay/defect more than one-half of affected middle cerebral artery territory remained until the late venous phase. DWI (J, K) and the collateral map (L–O) indicate that the core rapidly progresses to infarction. According to current guidelines, she should receive intravenous thrombolysis (IVT) after scanning NCCT (A, B). Even though the visually estimated NCCT ASPECTS is 5, it is not easy to exclude her from endovascular thrombectomy (EVT) because it is difficult to accept the NCCT ASPECTS. In addition, the automated ASPECTS is 7 (C), which is an eligible score for EVT. The result of automated software is dependent on the quality of the learning material, which is used as ground truth in deep learning. Automated ASPECTS is limited in accuracy due to the inconspicuousness of NCCT. We can see another result of the automated core size estimated with a threshold of CTP cerebral blood flow <30% (I), which is different from the results of visual and automated estimation. It is inevitably limited to measuring cores containing heterogeneous factors regarding occluded duration, collateral status, and cellular composition with different ischemic resistance as a fixed threshold value. This indirect method of core assessment is also due to the inconspicuousness of NCCT, and this is a major limitation of CT workup in acute ischemic stroke despite its availability and rapidity. However, DWI shows a far-progressed large infarction directly and clearly (J, K). We do not need to hesitate excluding her from EVT. At current guidelines, there is no basis for excluding her from IVT or for further imaging work-up except NCCT. We have no choice but to inject recombinant tissue plasminogen activator (rt-PA). However, through this case, we can understand the significant ratio of futile or dangerous treatments in current guidelines. |
Penumbra and mismatch concepts
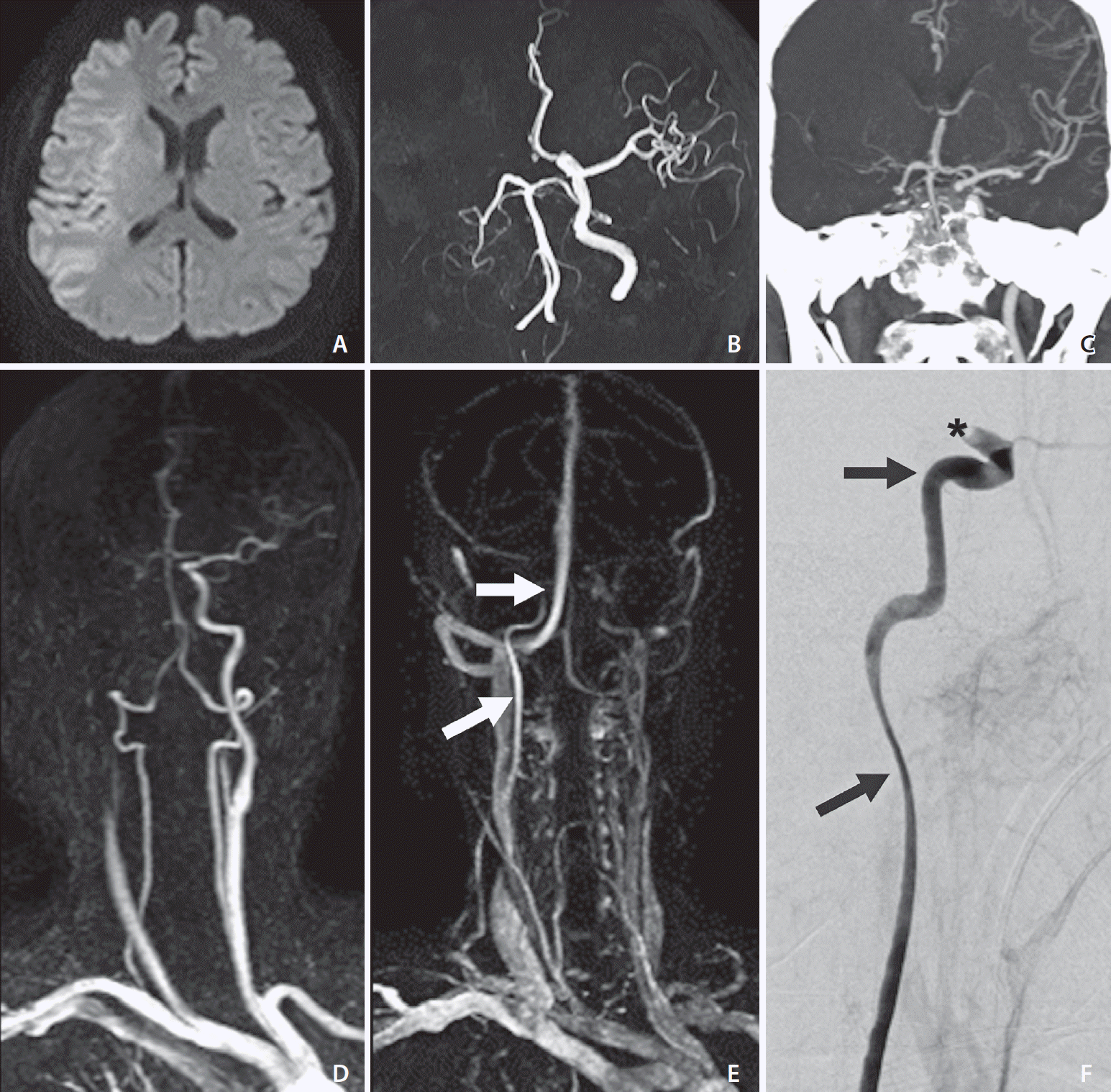 | Fig. 2.Images of an elderly patient who developed left-sided weakness 2 hours prior. (A) Diffusion-weighted magnetic resonance imaging at admission shows a core involving the whole right middle cerebral artery territory. (B) Time-of-flight magnetic resonance angiography (TOF-MRA) and (C) computed tomography angiography (CTA) show occlusion from the right proximal internal carotid artery (ICA) to the ipsilateral middle cerebral artery. (D) Early phase image of dynamic contrast-enhanced magnetic resonance angiography (DCE-MRA) shows the same finding as that of TOF-MRA and CTA. (E) More delayed phase image than (D) of DCE-MRA reveals delayed flow of the ICA reaching the cavernous segment (white arrows). (F) Catheter digital subtraction angiography reveals the patent right proximal ICA (black arrows) and occlusion of the C1 segment of the right ICA (*). |
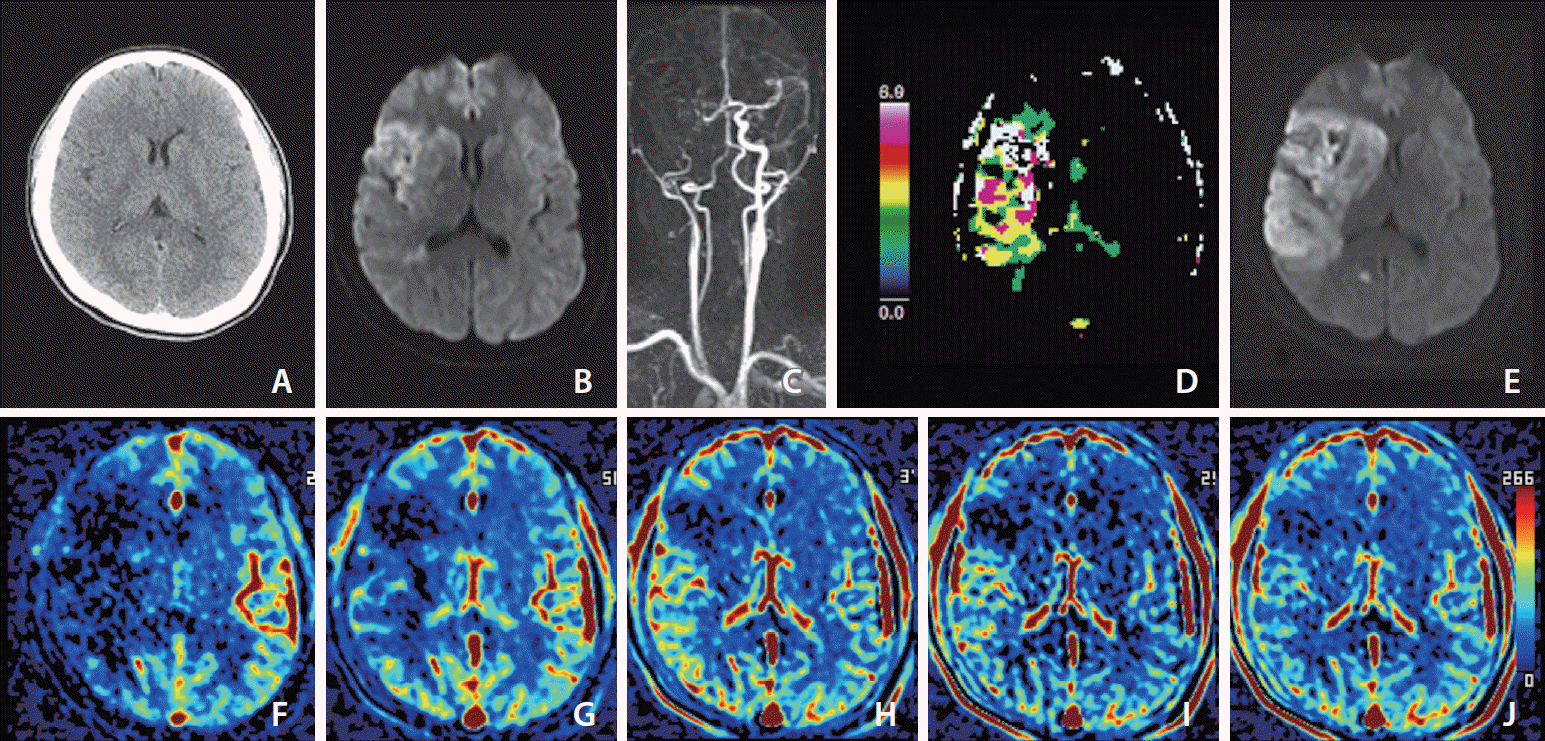 | Fig. 3.Images of a middle aged patient who developed left-sided weakness 30 minutes prior. Her premorbid modified Rankin scale score was 0, and the National Institutes of Health Stroke Scale score at admission was 11. She underwent intravenous thrombolysis followed by intraarterial thrombectomy, but recanalization of the occluded arteries was not achieved, as shown by a modified Thrombolysis in Cerebral Infarction scale score of 2a. (A) Noncontrast computed tomography image at admission shows less distinction of the right basal ganglia than the left basal ganglia. (B) Diffusion-weighted magnetic resonance imaging (DWI) at admission shows a core involving the insula, caudate nucleus, basal ganglia, internal capsule, M1, cortical area of M2 and M3, and some periventricular white matter of M3 on the right middle cerebral artery territory. (C) Dynamic contrast-enhanced magnetic resonance angiography (DCE-MRA) at admission shows occlusion of the right internal carotid and middle cerebral arteries. (D) MR perfusion Tmax threshold >6 seconds (Tmax >6 s) map at admission shows penumbra (white zone) matched with the core. (E) DWI on Day 1 shows a significantly increased extent of the core on DWI at admission due to reperfusion failure. (F–J) Images of arterial (F), capillary (G), early venous (H), late venous (I), and delay (J) phases of collateral map derived from DCE-MRA show intermediate to poor collateral perfusion status (MAC score of 2) [38] defined as collateral perfusion delay/defect more than one-half of affected middle cerebral artery territory in the capillary phase and equal to or less than one-half in the early venous phase. Because there was no target mismatch on Tmax >6 s (D), despite no penumbra on Tmax >6 s, the extent of the baseline core increased as much as the hypoperfused area on the capillary phase of the collateral map at admission, and the extent of the baseline core coincided with the hypoperfused area in the early venous phase. |
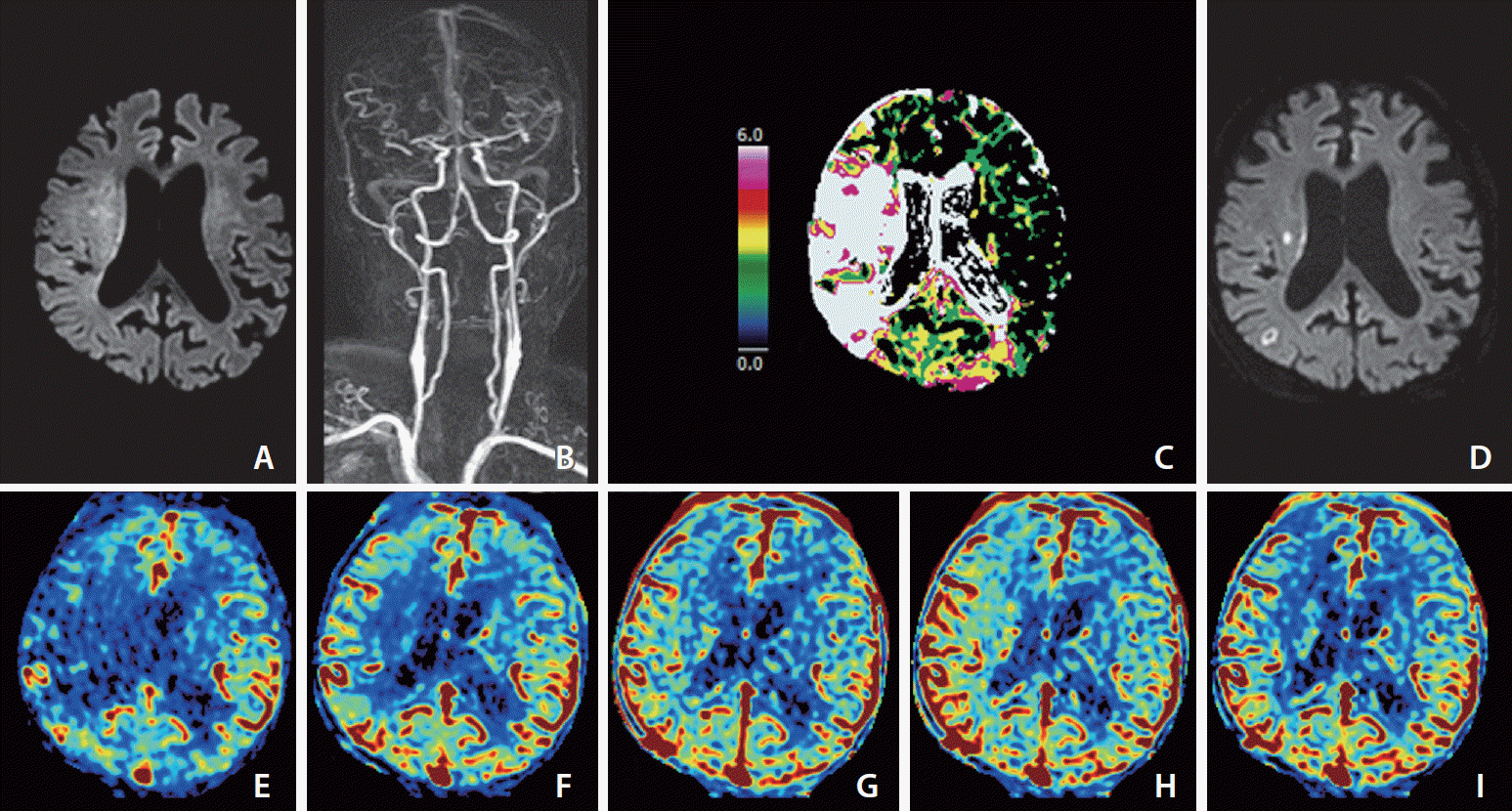 | Fig. 4.Images of an elderly patient who developed left-sided weakness 4 hours prior. His premorbid modified Rankin scale score was 0, and the National Institutes of Health Stroke Scale score at admission was 10. He was ineligible for IVT because he was taking an oral anticoagulant for arterial fibrillation. (A) Diffusion-weighted magnetic resonance imaging (DWI) at admission shows the core mainly involving more than one-third of the right middle cerebral artery (MCA) territory. (B) Dynamic contrast-enhanced magnetic resonance angiography (DCE-MRA) at admission shows partial occlusion at the first bifurcation of the right MCA. (C) MR perfusion Tmax threshold >6 seconds (Tmax 7>6 s) map at admission shows penumbra (white zone) larger than the core representing target mismatch. Catheter digital subtraction angiography performed prior to endovascular thrombectomy (EVT) revealed distal migration of fragmented thrombi to the M3 and M4 branches of the right MCA, so EVT did not undergo. (D) DWI on Day 1 shows that most of the baseline DWI lesions were reversed except for two tiny infarct signals. (E–I) Images of arterial (E), capillary (F), early venous (G), late venous (H), and delay (I) phases of collateral map derived from DCE-MRA show good collateral perfusion status (MAC score of 4) [38] defined as collateral perfusion delay equal to or less than one-half of affected MCA territory in the capillary phase and no or a small delay in the early venous
phase. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download