This article has been
cited by other articles in ScienceCentral.
Abstract
A novel endovascular technique to occlude high flow direct arteriovenous fistulae is presented, where the distal tip of the microcatheter acts as a nucleus that the operator can grow a plug from a liquid embolic agent. Its advantages (such as cost-saving and distal reachability), disadvantages (such as embolic material instability), and technique are discussed.
Keywords: Endovascular technique, Arteriovenous fistula, Interventional radiology
INTRODUCTION
Direct high flow fistulas represent a challenge during embolization because of the risk of embolic material being carried away to the venous system and perhaps landing in the lung to cause acute pulmonary embolism that potentially can cause cardiac arrest and death [
1]. This is especially problematic for liquid embolic material. Strategies to prevent this are numerous with varying degrees of appropriateness and effectiveness. We described a technique that is useful in selected cases, that can be employed with a non-adhesive liquid embolic agent. We believe it to be novel and can be added to the armament of other strategies used to overcome high flow arteriovenous fistulas.
Technical Description
Full anesthesia and paralysis are preferable. A microcatheter is navigated proximal (2–4 centimeters proximal depending on the vessel diameter, shape, and flow rate) to a direct fistula to decrease the odds of embolizing the vein before the arterial inflow is closed. An angiographic run is then performed in compliance with standard practice prior to embolization. If the microcatheter has excessive movement, it is advisable to reposition it to a less turbulent position, for instance where the distal tip of the microcatheter bumps into a vessel wall at a curve. Lowering the blood pressure is encouraged at this point. At this stage, a small amount of liquid embolic, preferably of the non-adhesive variety, is injected. The objective of the small volume is to be sufficiently small that the force that keeps it attached to the microcatheter is not exceeded by the drag force of the flowing blood. The 10–20 second wait is to allow the embolic agent time to solidify as it sets, and by that increase its force of attachment to the microcatheter (in the case of ONYX, by losing its dimethyl sulfoxide solvent in the blood stream, leaving behind the solidified/precipitated co-polymer). These steps are repeated to build an ever-enlarging catheter anchored embolic mass until the inflow is plugged, as summarized in
Table 1.
Consider an alternative technique if the tethered embolic material aggregate detaches prematurely after 1 or more attempts, depending on context and the operator’s best judgment. The authors intuitively without evidence feel that after 3 attempts the odds of success are slim.
CASE PEPORT
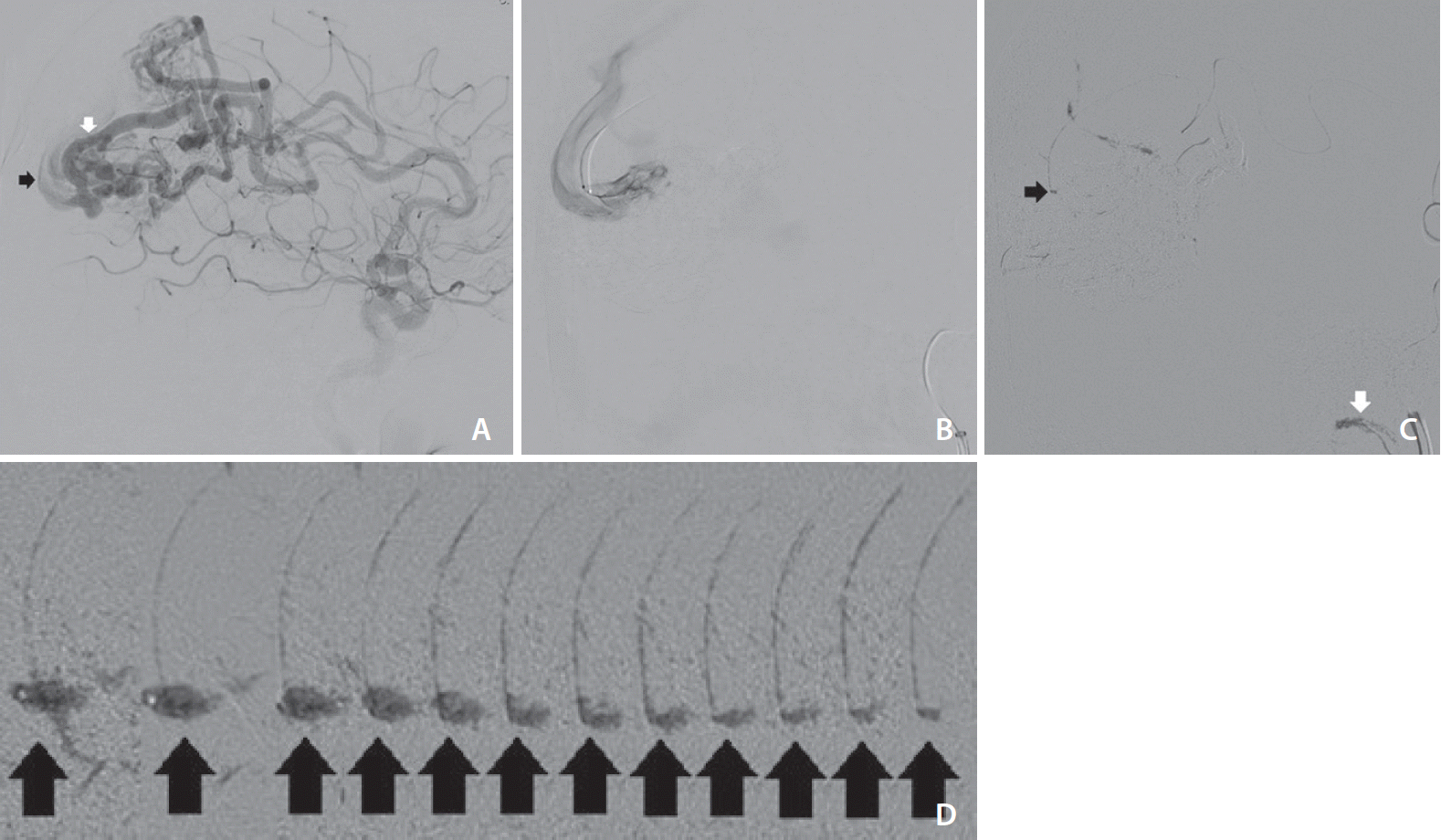
A patient presented to our center for treatment of a large (Spetzler-Martin grade IV–V) left occipital-parietal arteriovenous malformation with a nidus that harbors direct arteriovenous high flow shunts. The largest of these fistulae was from a hypertrophied left anterior cerebral artery feeder measuring approximately 5 mm in diameter (
Fig. 1A). A microcatheter (Apollo 1.5 French 5-centimeter detachable tip [eV3, Irvine, CA, USA]) was distally navigated to this feeder (
Fig. 1B). The initial injection of a non-adhesive liquid embolic agent (ONYX-18 [eV3]) was noticed to fly through the fistula unimpeded with some droplets reaching the jugular bulb (
Fig. 1C, white arrow). So, the standard injection technique (injections with visual stop cues) was abandoned in favor of the snowballing technique (injections with predetermined volume stop cues).
DISCUSSION
How the snowball grows is largely speculative. We presume that the non-adhesive liquid embolic agent at the distal tip of the microcatheter solidifies as it is exposed to blood and that the tip of the catheter offers a suitable surface where the non-adhesive liquid embolic agent can initially implant on. Then we presume that with each small incremental agent injection a new layer is added to the one before. Without a way for us to know for certain, it can be hypothesized that the new layer is added inside the injection before to cause it to grow (similar to how a multi-layer skin and mucosal layers are arranged, older is superficial and newer is deep), or and more likely, the new layer goes on top or outside the preceding layer (similar to how a snowball grows, older is central and newer is peripheral).
When the aggregate is small the dominant force acting on its molding is the blood flow. Notice that the initial right 7 images of the aggregated embolic agent in
Fig. 1D are pointing anteriorly and elongated, which happens to be the same force vector of blood flow. When the aggregate reaches a critical mass, gravity becomes a more important molding force, which accounts for the increased amount of embolic substance posterior to the microcatheter on the last left 5 images of
Fig. 1D.
This technique is not immune to criticisms. One such critic is the risk of premature detachment of a large aggregate that might be more harmful to the lung compared to the small embolic droplets used in the standard technique. Another disadvantage is the inherent instability of the snowball until a plug is achieved. There is also the additional time and radiation needed to execute this task. On the other hand, snowballing can be cost-saving as it may not necessitate using a balloon (e.g., balloon-assisted embolization) or placing large expensive embolic devices such as plugs or coils (e.g., pressure cooker technique) to slow down the flow [
2,
3]. It also might be the preferable option for fistulae that are only reachable with floppy microcatheters that cannot deliver non-liquid embolic options, and, therefore, alternative methods such as balloon-assisted embolization and the pressure cooker technique are not possible [
2,
3]. Additionally, this proposed predetermined volume stop cue technique can be used under a poor image quality environment when visual stop cue techniques are not suitable.
Hypothetically, the technique might be made more effective by using a larger diameter catheter as bigger objects offer a larger surface to implant the aggregate on. Also, greater viscosity embolic material should be less vulnerable to flow forces. Combining the snowball technique with other known techniques (e.g., blood pressure reduction or mechanical compression when feasible) is likely to have additive value.
The technique was used 3 times by the authors with no complications. Technical success was achieved in 2 and in 1 attempt it was abandoned for an alternative technique.
Conclusion
In conclusion, this technique may be used under poor image quality when visual stop cues are unfavorable. It may also be preferable in situations where non-liquid embolic agents cannot be delivered such as with the usage of floppy micro-catheters. This technique also has cost-saving benefits when compared to the usage of plugs or coils. A drawback to this technique is a higher potential harm from premature dislodgement due to the size of the embolic agent aggregate. In addition, execution of this technique requires more time and radiation. All in all, we believe this technique to have utility in the treatment of high-flow arteriovenous fistulas.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download