Introduction
Thoracic facet joint injection
1. Indication
2. Anatomy
3. Sonoanatomy and technique
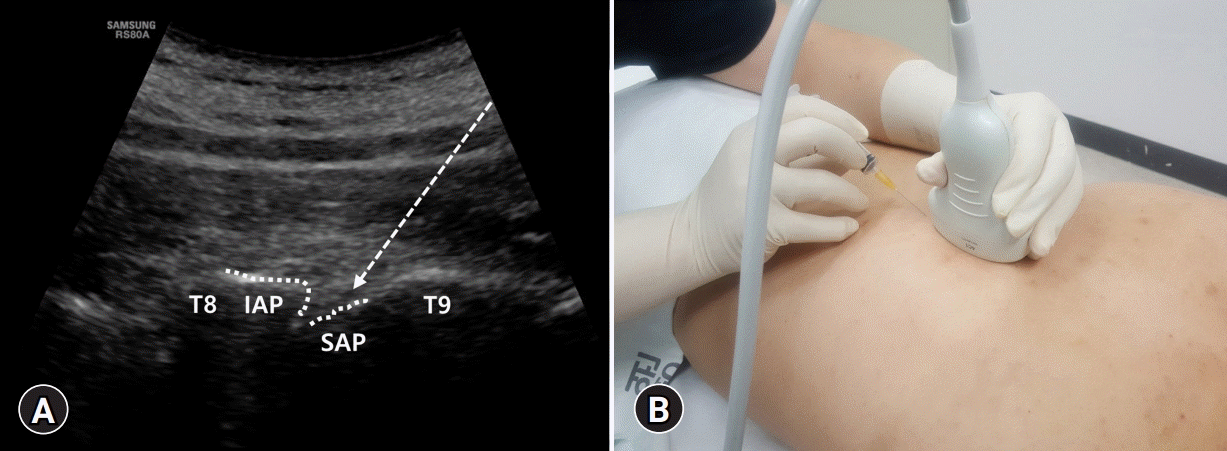 | Fig. 1.(A) The thoracic facet is formed by the union of the inferior articular process (IAP) and superior articular process (SAP). After the probe is slightly moved laterally over the lamina, between two hyperechoic lines, which are the IAP and SAP, the thoracic facet joint is visualized in the paramedian sagittal image. Dotted arrow indicates the pathway of the needle. (B) With the probe in one hand and the syringe in the other, insertion is performed longitudinally toward the proximal region, in-plane below 1- or 2-finger width below the probe. |
Costotransverse joint injection
1. Indication
3. Sonoanatomy and technique
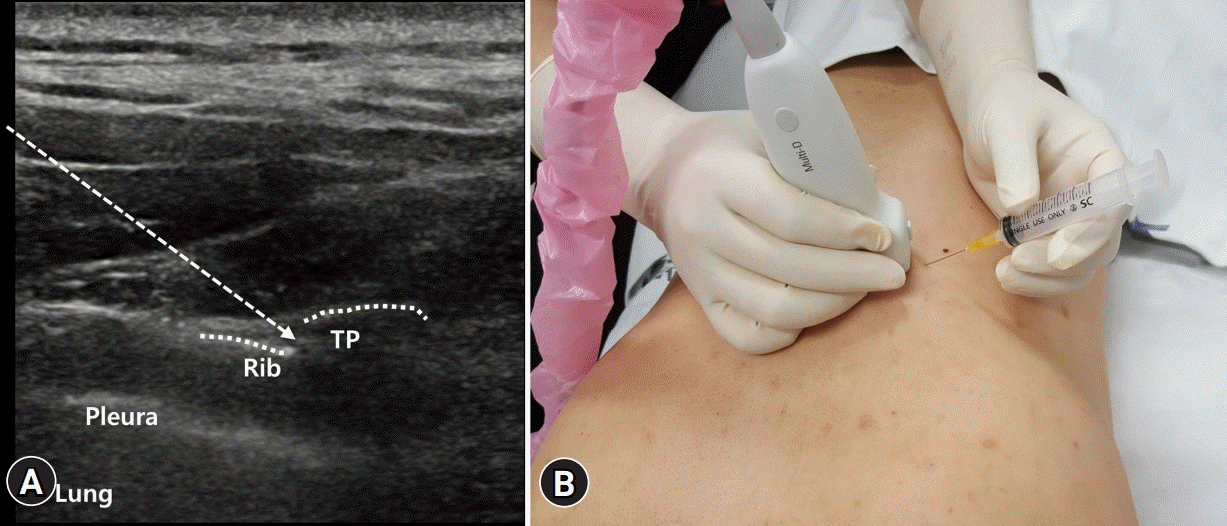 | Fig. 2.(A) The costotransverse joint is the articulation that connects the ribs with the transverse process (TP). Transverse scan is performed at the targeted thoracic level so that the TP, costotransverse joint, rib, and lung appear in the same plane. Dotted arrow indicates the pathway of the needle. (B) The injection is performed by an in-plane approach with the needle pointed toward the costotransverse joint in the lateral to medial direction. |
Thoracic paravertebral block
1. Indication
2. Anatomy
3. Sonoanatomy and technique
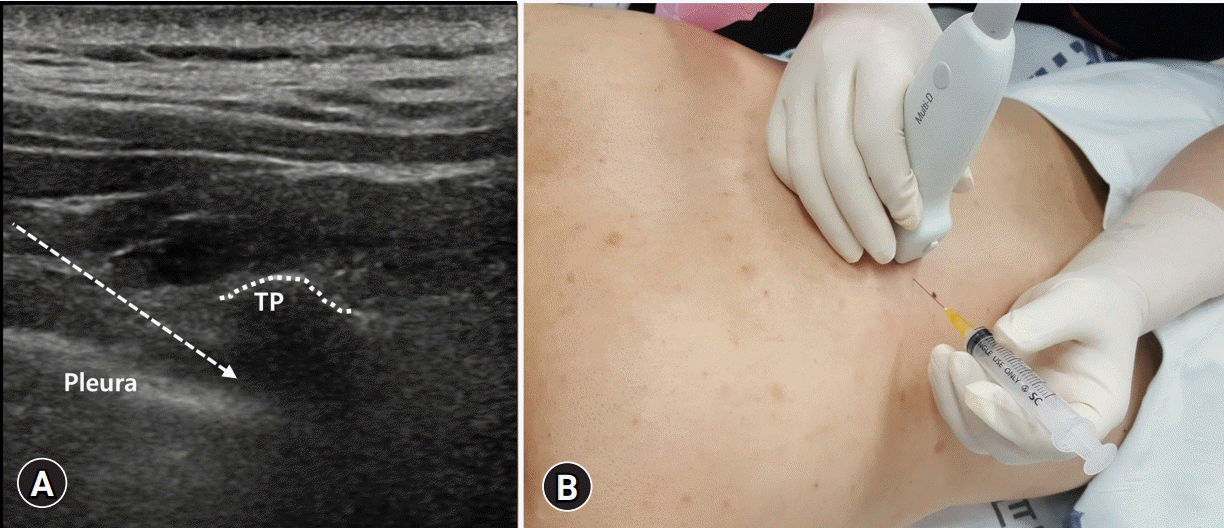 | Fig. 3.(A) The intercostal and thoracic paravertebral spaces are visible from the lateral and medial sides, respectively. The probe is fixed by the transverse scan in a position with a clear view of the thoracic paravertebral space, and the needle entry site is set to the lateral side of the probe for medial insertion. Dotted arrow indicates the pathway of the needle. (B) The needle tip is inside the thoracic paravertebral space, and the injection material is injected into the space. TP, transverse process. |
Intercostal nerve block
1. Indication
2. Anatomy
3. Sonoanatomy and technique
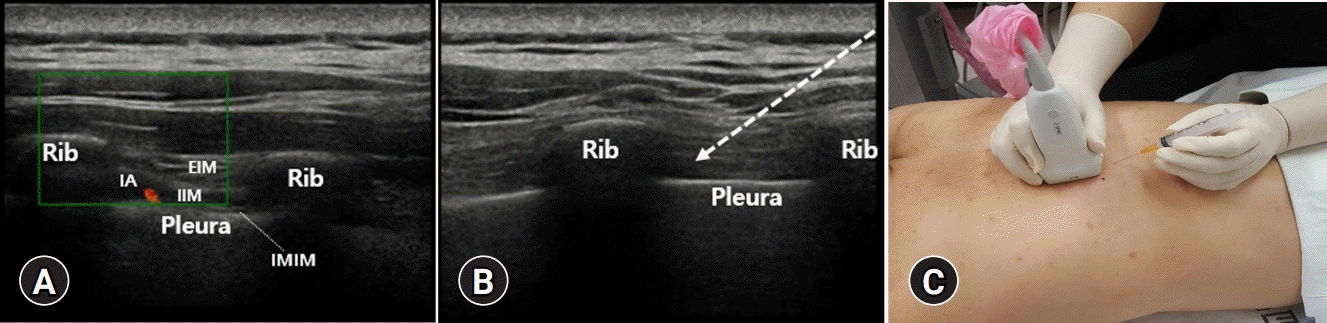 | Fig. 4.(A) In color Doppler, the intercostal artery (IA) within the main neurovascular bundle located in the lower margin of the rib is visible. (B, C) After finding the intercostal space, place the probe vertically, and advance the needle to the inferior margin of the rib. Dotted arrow indicates the pathway of the needle. EIM, external intercostal muscle; IIM, internal intercostal muscle; IMIM, innermost intercostal muscle. |
Erector spinae plane block
1. Indication
2. Anatomy
3. Sonoanatomy and technique
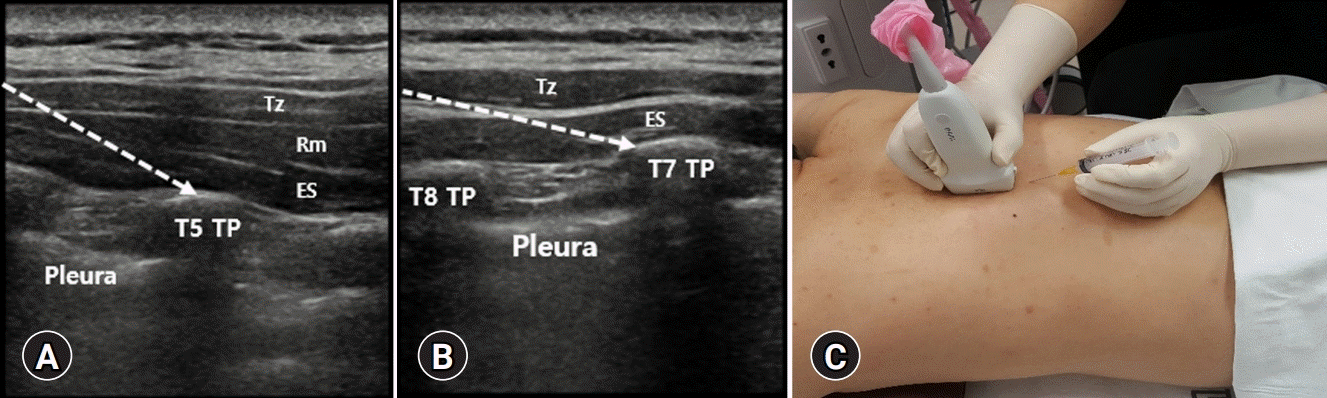 | Fig. 5.(A) At the T5 vertebral level, the following three muscles must be identified as above to the hyperechoic transverse process (TP) shadow: trapezius (Tz), rhomboid (Rm) major, and erector spinae (ES). (B) At the T7 vertebral level, however, the Rm major muscle disappears. The target of the injection must be in the fascial plane, deeper than the ES muscles at the tip of the TP of the thoracic spine. Dotted arrows indicate the pathway of the needle. (C) The needle is usually inserted into the plane. |
Pectoralis and serratus plane blocks
1. Indication
2. Anatomy
3. Sonoanatomy and technique
1) Pecs 1 block
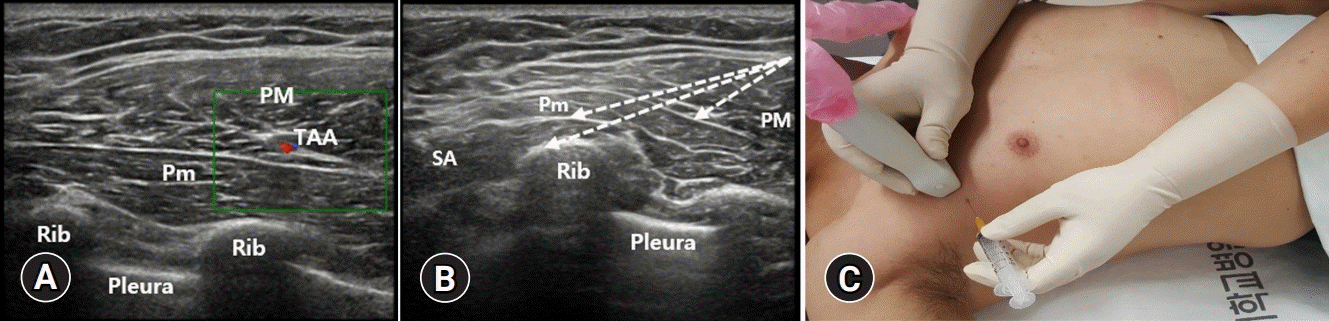 | Fig. 6.(A) The pectoral branch of the thoracoacromial artery (TAA), which is in close proximity to the lateral pectoral nerve, is seen as a small pulsating structure between the pectoral muscles, and it can be confirmed using the color Doppler ultrasound. (B, C) At the level between the 2nd and 4th ribs, the needle is introduced to the space under the serratus anterior (SA) muscle (Pecs 2 block). After injecting the anesthetic, the needle is pulled back to the interfascial space between the pectoralis minor (Pm) and the SA muscles under ultrasound guidance (Pecs 2 block). After injecting the anesthetic, the needle is pulled back to the interfascial space between the pectoral muscles where the Pecs 1 block is performed. Dotted arrows indicate the pathway of the needle. PM, pectoralis major. |
2) Pecs 2 block
3) Serratus plane block
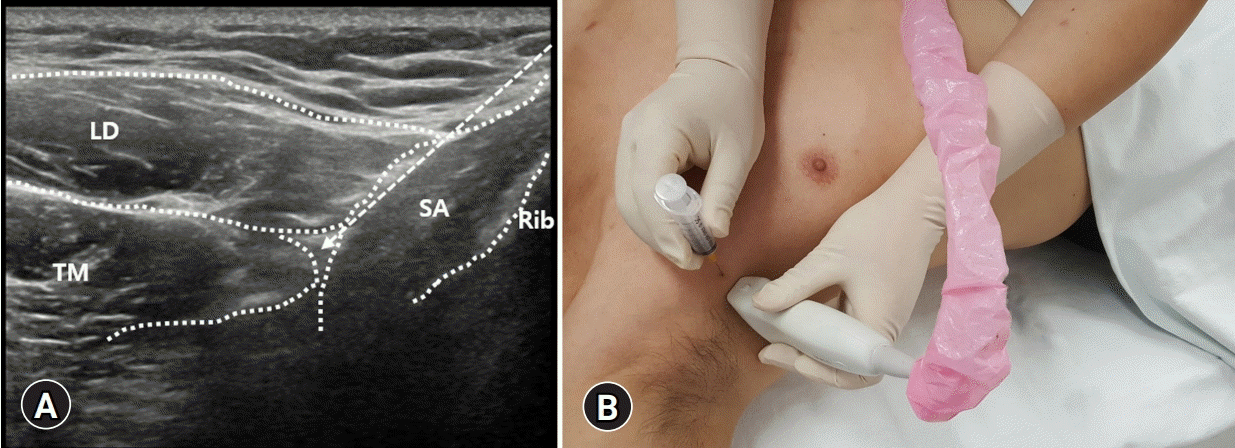 | Fig. 7.(A) The latissimus dorsi (LD) muscle is seen most superficially and posteriorly. The teres major (TM) is located superiorly deep to the LD muscle. Anteroinferiorly, the serratus anterior (SA) muscle is seen deep in the TM and LD muscles. The serratus plane block can be performed superficial or deep to the SA muscle. Dotted arrow indicates the pathway of the needle. (B) The probe is placed more laterally and posteriorly near the midaxillary to posterior axillary lines. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download