Abstract
Pain from nervous or musculoskeletal disorders is one of the most common complaints in clinical practice. Corticosteroids have a high pain-reducing effect, and their injection is generally used to control various types of pain. However, they have various adverse effects including flushing, hyperglycemia, allergic reactions, menstrual changes, immunosuppression, and adrenal suppression. Pulsed radiofrequency (PRF) is known to have a pain-reducing effect similar to that of corticosteroid injection, with nearly no major side effects. Therefore, it has been widely used to treat various types of pain, such as neuropathic, joint, discogenic, and muscle pain. In the current review, we outlined the pain-reducing mechanisms of PRF by reviewing previous studies. When PRF was first introduced, it was supposed to reduce pain by long-term depression of pain signaling from the peripheral nerve to the central nervous system. In addition, deactivation of microglia at the level of the spinal dorsal horn, reduction of proinflammatory cytokines, increased endogenous opioid precursor messenger ribonucleic acid, enhancement of noradrenergic and serotonergic descending pain inhibitory pathways, suppression of excitation of C-afferent fibers, and microscopic damage of nociceptive C- and A-delta fibers have been found to contribute to pain reduction after PRF application. However, the pain-reducing mechanism of PRF has not been clearly and definitely elucidated. Further studies are warranted to clarify the pain-reducing mechanism of PRF.
Most people experience pain due to pathologies of the nervous or musculoskeletal systems [1,2]. When the degree of pain is severe, patients’ quality of life decreases, and their function in daily activities and work deteriorates [1,2]. Therefore, pain control is important in clinical practice. Corticosteroid injections are frequently used [3,4]. However, they can have adverse effects including flushing, hyperglycemia, allergic reactions, menstrual changes, immunosuppression, and adrenal suppression [5,6]. To date, many injection materials have been suggested as substitutes for corticosteroids [7-9]. However, the effectiveness of materials that substitute corticosteroids is generally inferior to that of corticosteroids.
Electrical stimulation is thought to have a pain-reducing effect, and several types of electrical simulations have been used to alleviate pain [10-12]. Of these various stimulations, radiofrequency was found to have a pain-reducing effect similar to corticosteroid injection [13-15]. Continuous radiofrequency (CRF) exposes the target nerves or tissues to high temperatures (70°C–90°C) via continuous electrical stimulation [16]. Nerves or tissues treated with CRF are ablated [16]. The ablation of targeted nociceptive nerve fibers is thought to be the main mechanism of pain reduction after CRF [16]. However, as experiences of CRF use were accumulated, physicians realized that the pain could be effectively controlled even not under such high temperatures [17-19]. Therefore, it was thought that the formation and action of the electrical field around the target nerves or tissues would be a more important mechanism of PRF action than ablation by high temperatures. In 1998, Sluijter et al. [20] first introduced pulsed radiofrequency (PRF). By placing a long resting phase between brief electrical stimulation, PRF does not produce sufficient heat to cause structural damage [20]. Therefore, major complications rarely occur after PRF. Since its introduction, PRF has been widely used for various types of pain, such as neuropathic, joint, discogenic, and muscular pain [21-24] (Fig. 1).
To date, several clinical studies have demonstrated its positive analgesic effect [21-24]. In addition, many researchers have attempted to identify the mechanisms of action of PRF in alleviating pain. Although the exact mechanisms have not been elucidated, several possible mechanisms have been suggested.
This review aimed to outline the pain-reducing mechanisms of PRF by reviewing previous studies on this topic.
CRF supplies high-frequency continuous current to the targeted nerves [16]. The tip of the probe during the CRF procedure is at approximately 80°C and induces coagulative necrosis to target nerve structures around the probe tip [16]. Because the high temperature of a targeted structure decreases rapidly with distance from the electrode tip, lesions caused by the CRF procedure are well-circumscribed [16]. Therefore, other than damage to the targeted area, other tissues are rarely affected. Electrical neurolysis using CRF can inhibit the transfer of pain signals and has been proven to have a pain-reducing effect in various musculoskeletal disorders [25,26]. However, neurolysis can result in various side effects, such as sensory deficits, neuropathic pain, and skin burns [27,28].
In contrast, PRF uses a radiofrequency current comprising alternatively repeated electrical stimulation with a short duration (e.g., 20 ms) and resting phase (e.g., 480 ms) [17-20] (Fig. 2). This allows time for heat elimination and maintains the temperature of the target tissue below 42°C. Temperatures below 42°C rarely induce nerve tissue damage [17-24]. Therefore, adverse effects that can develop after the C-reactive protein procedure do not occur after the PRF procedure. PRF stimulation produces selective long-term depression (LTD) in C-fiber–mediated spinal sensitization [29,30]. LTD reduces the efficacy of neuronal synapses in C-fibers, and consequently, inhibits pain signaling from the peripheral nerve to the central nervous system [29,30]. LTD after PRF stimulation was supposed to be the main pain-reducing mechanism by Sluijter et al. [20], who invented the PRF procedure. Subsequently, several animal studies have been conducted to determine the pain-reducing mechanism of PRF, and these studies demonstrated that several mechanisms other than LTD are associated with pain reduction after the application of PRF (Table 1).
Microglia in the dorsal horn of the spinal cord play an important role in the induction and maintenance of neuroinflammation, resulting in chronic neuropathic pain [31,32]. Activated microglia release various inflammatory cytokines and chemokines that facilitate nociceptive processing at all levels of the neuraxis, including the spinal cord and supraspinal centers. Some previous animal studies have demonstrated the downregulation of microglia in rats with neuropathic pain after the application of PRF [31,32]. In 2013, Cho et al. [31] applied PRF stimulation (voltage, 45 V; pulse rate, 2 Hz; duration, 2 minutes) to the single dorsal root ganglion (DRG) in 23 Sprague-Dawley rats with sciatica due to herniated discs. After PRF application, mechanical withdrawal thresholds significantly increased, which persisted for 40 days. At 41 days after PRF application, microglia in the spinal dorsal horn were found to be deactivated. In 2016, Cho et al. [32] applied caudal epidural PRF (pulse rate, 5 Hz; pulse width, 5 ms; duration, 10 minutes) to 35 Sprague-Dawley rats with sciatica due to herniated discs. At 14 days post-PRF, in the sections of the spinal cord from L3, L4, L5, L6, and S1, microglial activation was attenuated in rats with herniated discs. The deactivation of microglia in the spinal dorsal horn after PRF application seems to prevent the progression from acute pain to chronic pain.
Inflammation is associated with acute and chronic neuropathic pain. An increase in proinflammatory cytokines, such as various types of interleukin (IL) and tumor necrosis factor-alpha (TNF-α), has been observed in the DRG and spinal dorsal horn in animal models of neuropathic pain [33,34]. In 2013, Vallejo et al. [35] evaluated the effect of PRF (voltage, 45 V; pulse width, 20 ms; duration, 3 minutes) on the ipsilateral L5 DRG in six rats exhibiting sciatic nerve injury. Following PRF therapy, increased proinflammatory gene expression, such as IL-6 and TNF-α, observed in the sciatic nerve and DRG of rats, returned to baseline values. Along with the decreased activation of proinflammatory gene expression, mechanical allodynia in the hind paw was alleviated. In 2019, Jiang et al. [36] applied PRF (pulse width, 20 ms; pulse rate, 2 Hz; duration, 2 minutes) on the ipsilateral L5 DRG or sciatic nerve in 20 rats with chronic constriction injury to the sciatic nerve. Mechanical allodynia and thermal hyperalgesia were relieved by PRF application. In addition, the authors found that IL-1β and TNF-α in the peripheral blood were downregulated. This anti-inflammatory effect of PRF appears to result in a reduction of various types of neuromuscular pain.
In 2012, Moffett et al. [37] investigated the molecular changes after applying PRF using cultured human dermal fibroblasts and human epidermal keratinocytes. After the application of PRF, the levels of endogenous opioid precursor messenger RNA (mRNA; proenkephalin, proopiomelanocortin, and prodynorphin) and corresponding opioid peptides were increased. This finding suggests that PRF exerts an analgesic effect by increasing endogenous opioid precursor mRNA levels.
Previous animal studies have demonstrated that the noradrenergic descending inhibitory pathway plays an important role in analgesic action [38]. In addition, activation of serotonin receptors, such as 5-HT1, 5-HT2, and 5-HT3, induces analgesic effects [39,40]. In 2009, Hagiwara et al. [41] performed an animal study in rats to evaluate the mechanism of PRF action. They induced unilateral hind paw hyperalgesia by injecting 0.15 mL of Freund’s complete adjuvant and applied PRF at 37°C or 42°C for 3 minutes on the sciatic nerves. The pain-reducing effect of PRF was significantly inhibited by intrathecal injection of the alpha2-adrenoceptor antagonist (yohimbine), the selective 5-HT3 serotonin receptor antagonist (MDL72222), and the nonselective serotonin receptor antagonist (methysergide). Based on their results, they suggested that the pain-reducing effect of PRF is correlated with the enhancement of the noradrenergic and serotonergic descending pain inhibitory pathways.
In 2017, Huang et al. [29] conducted experiments in rats with neuropathic pain induced by left L5 spinal nerve ligation. After PRF stimulation (pulse rate, 2 Hz; pulse width, 25 ms; duration, 5 minutes) on the left L5 DRG, the excitation of A- and C-afferent fibers was measured by checking the A- and C-components on the evoked field potential recordings. They found that PRF significantly suppressed the C-component overtime after 30 minutes, and this suppression was sustained for at least 140 minutes after PRF. However, the A component was not significantly suppressed after PRF stimulation. Mechanical allodynia and thermal analgesia significantly reduced after 10 and 14 days, respectively. This result indicates that PRF reduces neuropathic pain by inhibiting or suppressing the excitation of nociceptive C-fibers.
PRF is known to control pain without causing damage to the targeted tissue because the temperature of the targeted tissue does not exceed 42°C during PRF stimulation, and the threshold of tissue destruction is known to range from 45°C to 50°C. However, Erdine et al. [42] reported tissue destruction after PRF stimulation. In 2009, Erdine et al. [42] conducted PRF stimulation (voltage, 45 V; pulse rate, 2 Hz; pulse width, 1 ms) of the sciatic nerve of rats. The temperature was not allowed to exceed 42°C. The authors evaluated microscopic alterations in the nerve tissue using electron microscopy. After the application of PRF, the destruction of membranes, mitochondria, microfilaments, and microtubules was observed in the C-fibers, A-delta, and A-beta fibers. C- and A-delta fibers are nociceptive nerve fibers. The damage to these fibers was attributed to pain reduction after PRF stimulation.
In this review, we discuss previous studies on the mechanism of pain reduction using PRF. LTD of pain signaling from the peripheral nerves to the central nervous system, deactivation of microglia, reduction of proinflammatory cytokines, an increase in the endogenous opioid precursor mRNA, enhancement of descending pain inhibitory pathway, and inhibition and injury of nociceptive nerve fibers were suggested to contribute to pain reduction after PRF. However, the pain-reducing mechanism of PRF has not been clearly and definitely elucidated. Further studies are warranted to clarify the pain-reducing mechanism of PRF.
References
1. Chang MC. Conservative treatments frequently used for chronic pain patients in clinical practice: a literature review. Cureus. 2020; 12:e9934.

2. Choo YJ, Chang MC. Effectiveness of orthoses for treatment in patients with spinal pain. Yeungnam Univ J Med. 2020; 37:84–9.

3. Jang SH, Chang MC. Follow-up of at least five years after lumbar transforaminal epidural steroid injection for radicular pain due to lumbar disc herniation. Ann Palliat Med. 2020; 9:116–8.

4. Kwak DG, Kwak SG, Lee AY, Chang MC. Outcome of intra-articular lumbar facet joint corticosteroid injection according to the severity of facet joint arthritis. Exp Ther Med. 2019; 18:4132–6.

5. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002; 5:182–99.

6. Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009; 12:699–802.
7. Lai WF, Yoon CH, Chiang MT, Hong YH, Chen HC, Song W, et al. The effectiveness of dextrose prolotherapy in plantar fasciitis: a systemic review and meta-analysis. Medicine (Baltimore). 2021; 100:e28216.
8. Lee HJ, Ju J, Choi E, Nahm FS, Choe GY, Lee PB. Effect of epidural polydeoxyribonucleotide in a rat model of lumbar foraminal stenosis. Korean J Pain. 2021; 34:394–404.

9. Riewruja K, Phakham S, Sompolpong P, Reantragoon R, Tanavalee A, Ngarmukos S, et al. Cytokine profiling and intra-articular injection of autologous platelet-rich plasma in knee osteoarthritis. Int J Mol Sci. 2022; 23:890.

10. O’Connell NE, Ferraro MC, Gibson W, Rice AS, Vase L, Coyle D, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021; 12:CD013756.
11. Wu Y, Zhu F, Chen W, Zhang M. Effects of transcutaneous electrical nerve stimulation (TENS) in people with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2022; 36:472–85.

12. Yoon YS, Ko MH, Cho IY, Kim CS, Bajgai J, Jang HY, et al. Effects of personal low-frequency stimulation device on myalgia: a randomized controlled trial. Int J Environ Res Public Health. 2022; 19:735.

13. Kim TH, Chang MC. Comparison of the effectiveness of pulsed radiofrequency of the suprascapular nerve and intra-articular corticosteroid injection for hemiplegic shoulder pain management. J Integr Neurosci. 2021; 20:687–93.

14. Lee DG, Ahn SH, Lee J. Comparative effectivenesses of pulsed radiofrequency and transforaminal steroid injection for radicular pain due to disc herniation: a prospective randomized trial. J Korean Med Sci. 2016; 31:1324–30.

15. Lim JW, Cho YW, Lee DG, Chang MC. Comparison of intraarticular pulsed radiofrequency and intraarticular corticosteroid injection for management of cervical facet joint pain. Pain Physician. 2017; 20:E961–7.
16. Vatansever D, Tekin I, Tuglu I, Erbuyun K, Ok G. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain. 2008; 24:717–24.

17. Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42 degrees C to rat dorsal root ganglion and sciatic nerve. Spine (Phila Pa 1976). 2005; 30:1008–13.
18. Vallejo R, Benyamin RM, Kramer J, Stanton G, Joseph NJ. Pulsed radiofrequency denervation for the treatment of sacroiliac joint syndrome. Pain Med. 2006; 7:429–34.

19. West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: a case series. Pain Pract. 2010; 10:485–91.

20. Sluijter ME, Cosman ER, Rittmann WB, van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion: a preliminary report. Pain Clin. 1998; 11:109–17.
21. Boudier-Revéret M, Thu AC, Hsiao MY, Shyu SG, Chang MC. The effectiveness of pulsed radiofrequency on joint pain: a narrative review. Pain Pract. 2020; 20:412–21.

22. Chang MC. Efficacy of pulsed radiofrequency stimulation in patients with peripheral neuropathic pain: a narrative review. Pain Physician. 2018; 21:E225–34.
23. Yang S, Boudier-Revéret M, Chang MC. Use of pulsed radiofrequency for the treatment of discogenic back pain: a narrative review. Pain Pract. 2021; 21:594–601.

24. Yang S, Chang MC. Efficacy of pulsed radiofrequency in controlling pain caused by spinal disorders: a narrative review. Ann Palliat Med. 2020; 9:3528–36.

25. Conger A, Burnham T, Salazar F, Tate Q, Golish M, Petersen R, et al. The effectiveness of radiofrequency ablation of medial branch nerves for chronic lumbar facet joint syndrome in patients selected by guideline-concordant dual comparative medial branch blocks. Pain Med. 2020; 21:902–9.

26. Khan M, Meleka S. CT guided cervical medial branch block and radiofrequency ablation. J Clin Neurosci. 2020; 78:393–6.

27. Gazelka HM, Knievel S, Mauck WD, Moeschler SM, Pingree MJ, Rho RH, et al. Incidence of neuropathic pain after radiofrequency denervation of the third occipital nerve. J Pain Res. 2014; 7:195–8.

28. Stolzenberg D, Gordin V, Vorobeychik Y. Incidence of neuropathic pain after cooled radiofrequency ablation of sacral lateral branch nerves. Pain Med. 2014; 15:1857–60.

29. Huang RY, Liao CC, Tsai SY, Yen CT, Lin CW, Chen TC, et al. Rapid and delayed effects of pulsed radiofrequency on neuropathic pain: electrophysiological, molecular, and behavioral evidence supporting long-term depression. Pain Physician. 2017; 20:E269–83.
31. Cho HK, Cho YW, Kim EH, Sluijter ME, Hwang SJ, Ahn SH. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine. 2013; 19:256–63.
32. Cho HK, Kang JH, Kim SY, Choi MJ, Hwang SJ, Cho YW, et al. Changes in neuroglial activity in multiple spinal segments after caudal epidural pulsed radiofrequency in a rat model of lumbar disc herniation. Pain Physician. 2016; 19:E1197–209.
33. Leung L, Cahill CM. TNF-alpha and neuropathic pain: a review. J Neuroinflammation. 2010; 7:27.
34. Hung AL, Lim M, Doshi TL. Targeting cytokines for treatment of neuropathic pain. Scand J Pain. 2017; 17:287–93.

35. Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013; 16:E601–13.
36. Jiang R, Li P, Yao YX, Li H, Liu R, Huang LE, et al. Pulsed radiofrequency to the dorsal root ganglion or the sciatic nerve reduces neuropathic pain behavior, decreases peripheral pro-inflammatory cytokines and spinal β-catenin in chronic constriction injury rats. Reg Anesth Pain Med. 2019; 44:742–6.

37. Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res. 2012; 5:347–57.

38. Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014; 8:143–51.

39. Diniz DA, Petrocchi JA, Navarro LC, Souza TC, Castor MG, Perez AC, et al. Serotonin induces peripheral mechanical antihyperalgesic effects in mice. Eur J Pharmacol. 2015; 767:94–7.

40. Jeong HJ, Mitchell VA, Vaughan CW. Role of 5-HT(1) receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br J Pharmacol. 2012; 165:1956–65.
Fig. 2.
The waveforms of continuous RF (CRF) and pulsed RF (PRF). While CRF is applied continuously without any resting phase, PRF has a long resting phase between brief electrical stimulation. RF, radiofrequency; Voltage, the amplitude of pulsed RF current.
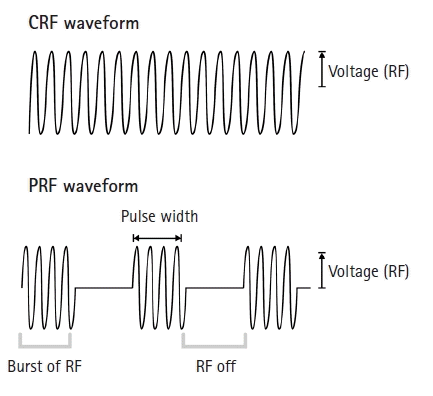
Table 1.
The suggested pain-reducing mechanism of pulsed radiofrequency




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download