This article has been
cited by other articles in ScienceCentral.
Abstract
Nephrocalcinosis often occurs in infants and is caused by excessive calcium or vitamin D supplementation, neonatal primary hyperparathyroidism, and genetic disorders. Idiopathic infantile hypercalcemia (IIH), a rare cause of nephrocalcinosis, results from genetic defects in CYP24A1 or SLC34A1. Mutations in CYP24A1, which encodes 25-hydroxyvitamin D 24-hydroxylase, disrupt active vitamin D degradation. IIH clinically manifests as failure to thrive and hypercalcemia within the first year of life and usually remits spontaneously. Herein, we present a case of IIH wih CYP24A1 mutations.
An 11-month-old girl visited our hospital with incidental hypercalcemia. She showed failure to thrive, and her oral intake had decreased over time since the age of 6 months. Her initial serum parathyroid hormone level was low, 25-OH vitamin D and 1,25(OH)2 vitamin D levels were normal, and renal ultrasonography showed bilateral nephrocalcinosis. Whole-exome sequencing revealed compound heterozygous variants in CYP24A1 (NM_000782.4:c.376C>T [p.Pro126Ser] and c.1310C>A [p.Pro437His]). Although her hypercalcemia and poor oral intake spontaneously resolved in approximately 8 months, we suggested that her nephrocalcinosis and renal function be regularly checked in consideration of potential asymptomatic renal damage. Hypercalcemia caused by IIH should be suspected in infants with severe nephrocalcinosis, especially when presenting with failure to thrive.
Keywords: Case reports, Failure to thrive, Hypercalcemia, Nephrocalcinosis, Vitamin D3 24-hydroxylase
Introduction
Nephrocalcinosis, a possible cause of chronic kidney disease (CKD), is rarely identified during infancy. Because it may lead to kidney damage [
1,
2], the underlying causes need to be identified and managed, if possible. Nephrocalcinosis is commonly caused by primary hyperparathyroidism, long-term use of loop diuretics or vitamin D, distal renal tubular acidosis, and hereditary disorders, such as Bartter syndrome [
1,
3]. Therefore, evaluation of childhood nephrocalcinosis includes urine analysis of hematuria, protein excretion, pH, calcium excretion, and other minerals such as uric acid, oxalic acid, phosphate, and citrate. Analyses of serum calcium, phosphorus, magnesium, uric acid, alkaline phosphatase, pH, bicarbonate, and creatinine levels are also required. Additional studies including parathyroid hormone (PTH), vitamin D metabolites, and molecular genetic testing should be considered [
4]. When nephrocalcinosis is associated with hypercalcemia and/or hypercalciuria, clinicians should consider the possibility of a genetic disorder, such as Williams syndrome, Jansen’s metaphyseal chondrodysplasia, or blue diaper syndrome [
5].
Idiopathic infantile hypercalcemia (IIH) is a rare disorder caused by a genetic defect in the key enzymes involved in calcium and vitamin D metabolism. The incidence of IIH is approximately 1 in 33,000 live births, and until now, two causative genes have been identified for IIH:
CYP24A1 (IIH type 1) and
SLC34A1 (IIH type 2) [
6].
CYP24A1 encodes 25-hydroxyvitamin D-24-hydroxylase (CYP24A1), a component of the mitochondrial inner membrane P450. When vitamin D
3, cholecalciferol, is administered to the human body through the skin and diet, it is metabolized through 25-hydroxylation in the liver and then via 1a-hydroxylation in the kidney to produce biologically active 1,25-dihydroxyvitamin D
3 [1,25-(OH)
2D
3]. This active 1,25-(OH)
2D
3 is catabolized by CYP24A1, making it a water-soluble metabolite of calcitroic acid [
7]. CYP24A1 also catabolizes 25-(OH)D
3, the precursor of active vitamin D. Thus,
CYP24A1 plays a critical role in regulating active vitamin D levels, and defective CYP24A1 increases the concentration of 1,25-(OH)
2D
3 in the blood.
SLC34A1 encodes the Na+/phosphate (Pi) cotransporter NaPi-IIa, a transmembrane cotransporter in the proximal renal tubule, which plays an essential role in absorbing Pi from primary urine [
6]. Defective absorption of Pi due to dysfunctional NaPi-IIa in IIH type 2 stimulates inappropriate synthesis of 1,25-(OH)
2D
3. Therefore, both IIH types 1 and 2 present with an inappropriately high concentration of 1,25-(OH)
2D
3, calcitriol. Calcitriol regulates serum ionized serum calcium levels by stimulating intestinal calcium reabsorption and renal calcium reabsorption at the distal tubule (along with PTH) or activating PTH when serum calcium levels are low. PTH stimulates osteoclast formation and differentiation, which in turn induces calcium mobilization from bones [
8]. Therefore, in IIH, abnormally elevated active calcitriol levels eventually cause hypercalcemia via its action on the intestine, kidneys, and bones.
In patients with IIH, the classic manifestations include vomiting, anorexia, polyuria, polydipsia, hypotonia, and failure to thrive within 1 year of life, most commonly within 3 to 7 months of age. Unexplained fever, constipation, and hypertension can also occur. Laboratory evaluations usually reveal suppressed serum PTH levels, mildly elevated 25-(OH)D
3 and 1,25-(OH)
2D
3 levels, and hypercalciuria, often accompanied by nephrocalcinosis [
7,
9,
10]. The management of IIH is mainly conservative, with a recommendation of low-calcium diet and avoiding excessive vitamin D. When necessary, intravenous fluid hydration can be used to alleviate hypercalcemia and dehydration. If symptomatic hypercalcemia persists, glucocorticoids to prevent renal calcium reabsorption and inhibition of 1,25-(OH)
2D
3 activity, bisphosphonates for inhibition of osteoclast activity, or azole agents (e.g., ketoconazole) for inhibition of P450 enzymes can be considered. Pi supplementation is necessary in case of defective NaPi-IIa in IIH type 2 [
6]. In rare cases, rapid hemodialysis management is required to treat life-threatening hypercalcemia [
6,
9,
11].
Herein, we present a case of severe nephrocalcinosis, wherein genetic analysis revealed IIH type 1 with pathogenic compound heterozygous variants of CYP24A1. This report has its significance in that this case is the first report of IIH type 1 patient of South Korea, among whom genetic analysis had been done.
Case report
An 11-month-old girl was transferred to our hospital for a second opinion on incidentally discovered hypercalcemia. Hypercalcemia was found at a primary hospital during laboratory workup for fever. Initially, her calcium level was 13.4 mg/dL (reference: 8.6–10.2 mg/dL), ionized calcium level was 1.45 mmol/L (reference: 1.15–1.33 mmol/L), and spot urine calcium to creatinine was 2.19 mg/mg (reference: ≤0.6 mg/mg creatinine), respectively. Serum phosphate level was 5.0 mg/dL, and blood urea nitrogen and creatinine were 23.8 mg/dL and 0.44 mg/dL, respectively. The albumin level was normal and intact PTH level was low, less than 0.7 pg/dL. The urine analysis at the primary hospital showed hypercalciuria, but there was no hematuria nor proteinuria. Urine electrolytes (sodium, potassium, uric acid, phosphorus, and magnesium) were not abnormally elevated.
When she was initially brought to our hospital, physical examination results were nonspecific and unremarkable. The fever had subsided, but poor oral intake, which was noticed at the age of 6 months, persisted. At that time, her serum calcium and phosphorus levels were 12.2 mg/dL (reference: 8.8–10.5 mg/dL) and 4.7 mg/dL (reference: 4.1–6.2 mg/dL), respectively. Ionized calcium level was 1.62 mmol/L (reference: 1.05–1.35 mmol/L), and creatinine level was 0.54 mg/dL. Serum albumin level was 4.7 g/dL (reference: 3.3–5.2 g/dL). Urine analysis revealed no hematuria or proteinuria, and urine calcium to creatinine ratio also improved so there was no hypercalciuria. Her spot urine calcium to creatinine ratio was 0.36 mg/mg.
She was born after 37 weeks and 4 days of gestation, with a birth weight of 3.2 kg (62nd percentile). She had no developmental delays. She had been fed approximately 600 mL/day of powdered milk and 200 mL/day of weaning food. She was receiving 5 mL/day of multivitamin supplementation containing cholecalciferol (4,000 IU/100 mL).
Further evaluations for hypercalcemia revealed low serum PTH levels (0.7 pg/mL, reference: 8–76 pg/mL) excluding hyperparathyroidism, normal PTH-related peptide (less than 1.1 pmol/L), 25(OH) vitamin D (46.7 ng/mL, reference: 30–100 ng/mL), and 1,25(OH)
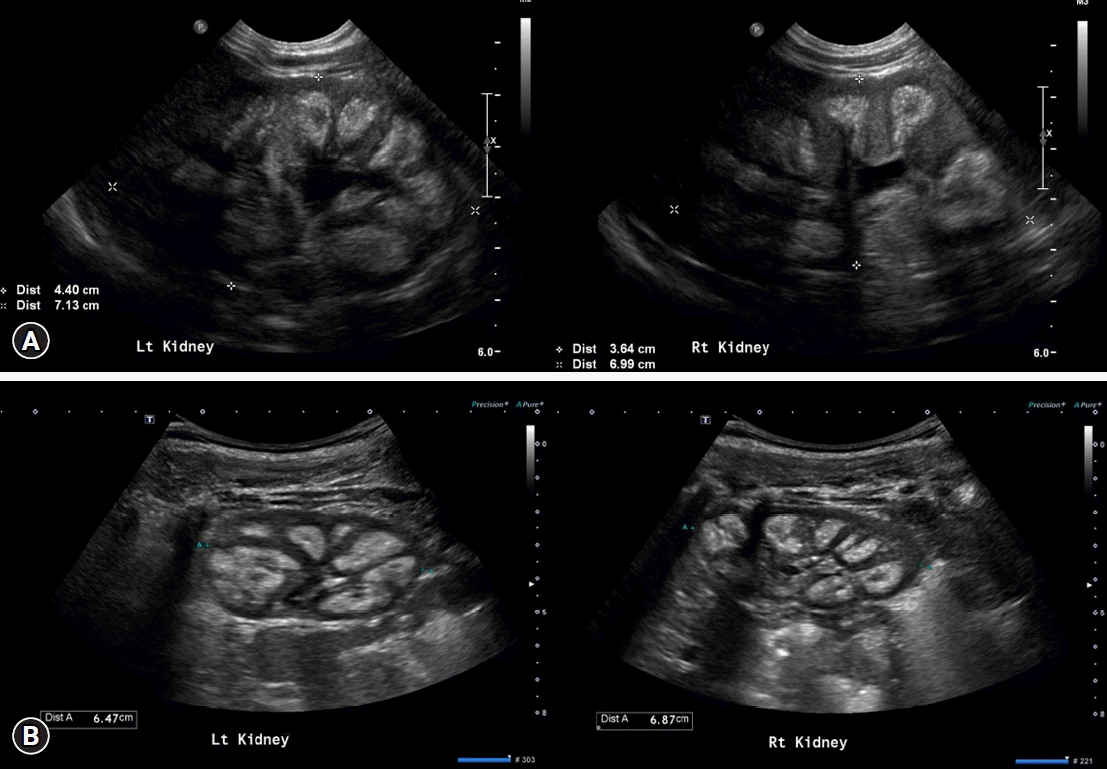
2 vitamin D levels (23.4 pg/mL, reference: 19.6–54.3 pg/mL). Kidney ultrasonography revealed diffusely increased echogenicity of the medullary pyramids, indicating nephrocalcinosis (
Fig. 1A).
Echocardiography was performed to rule out Williams syndrome; however, the findings were unremarkable. Whole-exome sequencing was carried out from genomic DNA extracted from buccal swab sample, by company named “3 billion." The test result revealed compound heterozygous variants of uncertain significance in CYP24A1 (NM_000782.4), c.376C>T (p.Pro126Ser), and c.1310C>A (p.Pro437His), which were predicted in silico to be pathogenic, and likely damaging to the protein structure or function. These variants were reported to have an extremely low frequency in both the gnomAD v2.1.1 and v3 datasets.
We advised the patient’s parents that the multivitamin supplementation be discontinued and additional calcium supplementation be avoided for her. We decided to observe her clinical progression without any other additional management, and her serum calcium levels spontaneously decreased over time, improving oral intake. After 2 months, her serum calcium levels were 10.2 mg/dL with ionized calcium levels at 1.39 mmol/L. At the last visit, which was after 8 months from the first visit to our hospital, serum calcium levels and ionized calcium levels were 10.1 mg/dL and 1.33 mmol/L, respectively. Her body weight at the initial visit to our hospital was 7.1 kg, which was less than the 3rd percentile for her age. After 2 months, her body weight was 9.3 kg, which was in the 25th to 50th percentile for her age, and her height was 73 cm, which was in the 5th to 10th percentile. During the last follow-up at the age of 21 months, the patient’s body weight and height were 12 kg (75th–90th percentile) and 85.3 cm (50th–75th percentile), respectively. Follow-up kidney ultrasonography was performed 9 months after the visit, and bilateral kidney nephrocalcinosis was found to be still present (
Fig. 1B).
Discussion
Herein, we have discussed a typical case of IIH type 1, presenting with severe nephrocalcinosis associated with hypercalcemia and normophosphatemia. Other case reports of IIH in South Korea were those of IIH type 2 (
SLC34A1 mutation) [
12,
13]. In one report, nephrocalcinosis was prenatally detected at 28 weeks of gestation. The patient was treated with intravenous hydration, furosemide, and corticosteroids for hypercalcemia (12.8 mg/dL), which improved within 7 days. The patient was fed a low-calcium, vitamin D-free formula, and at the age of 7 months, renal echogenicity also improved [
12]. This case report differs from our index patient in that hypercalcemia and nephrocalcinosis improved more rapidly with more aggressive treatment. Additionally, the genetic causes were different. Theoretically, serum phosphorus levels are expected to be low in IIH type 2, as shown in a study using a mouse model, wherein the serum phosphorus levels were not normalized by vitamin D restriction, but only by Pi load [
6]. However, one of the previous Korean reports of IIH and our case involved normophosphatemia, making a differential diagnosis between the two types of IIH challenging, as previously reported. The 1,25-(OH)
2D
3 level in our case was within the normal range, similar to that in previous reports. We suppose that it should have been lower than normal if our patient’s CYP24A1 enzyme was not defective since she had severe hypercalcemia, which would have reduced the 1,25-(OH)
2D
3 level through negative feedback.
In addition to the incidental fever, the chief complaint in our case was poor oral intake. This is a common symptom of IIH. As hypercalcemia impairs gastrointestinal motility via reduction in the contractility of smooth muscles, this may contribute to delay of gastric emptying and subsequent reduced appetite [
14]. Furthermore, hypercalcemia promotes gastrin secretion, which may contribute to nausea and poor oral intake [
15]. Thus, if infants with nephrocalcinosis exhibit poor oral intake, hypercalcemia should be considered, of which IIH is a rare cause. Other more common causes of infantile hypercalcemia include vitamin D overdose and Williams syndrome. Therefore, workup of serum vitamin D metabolites and echocardiography screening are necessary to clinically exclude these etiologies and suspect IIH. Since IIH remits spontaneously in many cases, radical management might not be necessary, and close follow-up is indicated, as shown herein. Nonetheless, long-term follow-up, including kidney function, is necessary because there is a chance of ongoing subclinical metabolic abnormalities in these patients.
The natural history and long-term outcomes of IIH are not well known. Clinical symptoms seem to disappear spontaneously with the normalization of serum calcium levels. However, one study involving long-term follow-up of 18 patients with IIH showed that the renal prognosis of survivors of IIH tended to be poorer than that of the general population, demonstrating a high prevalence of CKD, with CKD II in 77% and two cases of end-stage kidney failure despite avoidance of vitamin D or calcium supplementation and sun exposure [
10].
A limitation of this report is that phasing of the variants was not possible in this case. Thus, we are not sure whether the two variants exist as cis or trans isomers. In addition, these mutations are variants of unknown significance and have not been classified to be pathogenic or likely pathogenic according to ACMG (American College of Medical Genetics and Genomics) guidelines. Although, the whole-exome sequencing result of the patient met 1 criterion of “moderate evidence of pathogenicity,” and 2 criteria of “supporting evidence of pathogenicity.” Firstly, these variants have been reported with an extremely low frequency from gnomAD dataset. Secondly, in silico prediction tools and conservational analysis supported deleterious effects on the gene product. Thirdly, the patient’s phenotype is highly specific for IIH. The patient’s clinical presentation and course are compatible with those of IIH type 1, suggesting that this rare disease needs to be considered.
We reported a case of IIH caused by caused by CYP24A1 mutations that presented with fever, failure to thrive, and severe nephrocalcinosis. When idiopathic hypercalcemia and poor oral intake or nephrocalcinosis are present, consideration of IIH as a causative disease is necessary. A proper and timely diagnosis of this disease can help in the correct management. Although the patient’s hypercalcemia resolved, her nephrocalcinosis persisted, implying that careful regular check-ups for her kidney status are needed as subclinical renal injury may still progress.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download